Introduction
Radiation therapy (RT) is used to reduce the risk of relapse after surgery, as a means for reduction of tumor volume in palliative care, or as the main method of treatment for some tumors. As a rule, RT for cancer patients involves local irradiation of the tumor node. However, the researchers have been observed cases of ŌĆ£distantŌĆØ effects of radiotherapy, in particular regression of hematogenous metastases, with local irradiation of the primary tumor node since the beginning of the 20th century.
The first complete regression of the tumor was noted in 1908 when the effect of radiation was studied on lymph nodes remote from the main focus. Unfortunately, in this study there was no exact description of the irradiation procedure, dose, and localization of the irradiated lymph nodes, which cannot exclude the influence of other factors on the reached effect [1]. In 1938, an ŌĆ£irregular reactionŌĆØ (adrenal hypertrophy and atrophy of lymphoid organs) was observed in unirradiated organs in animal model in vivo, when a tumor was irradiated 20ŌĆō40 Gy [2]. Fifteen years later, in 1953, Mole [3] suggested introducing the term ŌĆ£abscopal effectŌĆØ to denote the effect of ionizing radiation ŌĆ£at a distance from the irradiated volume but within the same organism,ŌĆØ and then expanded to include distant effects on normal tissues [4]. Currently, it is a hypothesis in the treatment of metastatic cancer, when there is a regression of untreated areas simultaneously with a decrease in the tumor. This means that localized radiation initiated an antitumor response, which kills cancer cells remote from the main target. Despite the fact that the first recorded clinical cases of tumor regression after chemotherapy and RT date back to the beginning of the 20th century, the abscopal effect is still quite rare in clinical practice [4].
It is important to note that cases of tumor regression were also observed in cancer patients with various infectious diseases [5]. American surgeon Coley [6] was convinced that having a severe infection could cause tumor regression. His experiments showed that the inactivated pathogens of Streptococcus pyogenes in the vaccinated patient could not cause an infectious disease, but nevertheless all the signs of inflammation developed, and the body began to fight and the tumor disappeared. Interestingly, in experiments in vitro, bacterial strains did not affect tumor cells in any way. Therefore it was concluded that the infection did not directly impact on the tumor, but activated the patient's immunity, which led to the regression of the tumor node.
Scientists have argued over the role of the immune system in the antitumor response over a long period of time. More than 30 years ago, Stone et al. [7] showed that there is a lack of tumor response to RT with the deficiency of a normal pool of T cells. It is now well known that tumor progression is associated with the development of many immunosuppressive mechanisms that allow cancer cells to escape immune control. Consider that RT also has an immunosuppressive effect and is not able to induce an effective antitumor immune response, which leads to the destruction of the tumor. However, in some cases, immunosuppression and the development of an antitumor immune response as an abscopal effect. In this case, stimulation of the patientŌĆÖs immunity (through immunotherapy) and/or overcoming of tumor-induced immunosuppression during RT should provide an increase in the latterŌĆÖs effectiveness and an increase in the frequency of the abscopal effect.
Mechanism of the Abscopal Effect
It is believed that RT has an immunosuppressive effect, leads to the suppression of co-stimulatory surface markers CD80 and CD86 on native dendritic cells, thereby inhibiting the activation of T cells [8]. However, cell death caused by RT stimulates many immune responses and changes the tolerant tumor phenotype [9]. During radiation therapy, a special functional type of cell apoptosis can be activatedŌĆöimmunogenic cell death (ICD), which is accompanied by stimulation of antigen-specific adaptive immunity [10]. ICD leads to many antitumor immune responses: the release of tumor antigens by irradiated tumor cells, the cross-presentation of antigens originating from the tumor to T cells using antigen-presenting cells, and the migration of effector T cells from lymph nodes to distant tumor [11]. In this regard, the number of studies on the effect of RT on various immune responses (activation of antitumor immune responses) and the abscopal effect has increased. In a recent review that covered the time period from 1969 to 2014, 46 cases of the demonstration of an abscopal effect in patients after RT were described [12]. Moreover, the frequency of occurrence of the RT-induced abscopal effect is very low [13]. However, the documented occurrence of this effect in RT provides an incentive for a more thorough further study.
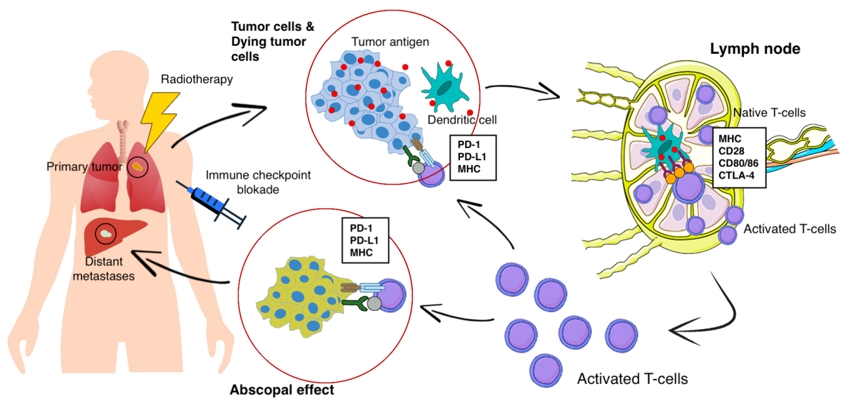
At present, the following data on the mechanisms of the abscopal effect are available. When exposed to tumor cells in RT, the dying tumor cells have a huge number of changes and begin to actively express damage-associated molecular patterns (DAMP), calreticulin, adenosine triphosphate, high mobility group box 1 (HMGB1) protein, type I interferons, nucleic acids derived from cancer cells, annexin A1, etc. [14], releasing a large number of tumor antigens (Fig. 1). Antigen-presenting cells, such as dendritic cells and phagocytic cells, interact with the emerging tumor antigens and then migrate to the lymph nodes. The presentation of antigens occurs through the major histocompatibility complex (MHC), through the T cell receptor (TCR) [15]. However, antigenic complex interactions only through the TCR are not enough to activate T cells. Other co-stimulating signals are needed, such as CD80, CD86, and CD28 [16]. If activation by several T cell signals occurred (there was no inhibition on the part of the tumor), then many types of T cells and, especially, CD8+ T cells, which play the main role in the antitumor immune response, are activated and begin to reproduce. As a result, activated effector T cells exit the lymph nodes, and interactions with TCR affect the primary tumor and unirradiated distant metastasis [16]. Presumably, such a mechanism can be used to explain the abscopal effect in distant metastases with local tumor irradiation. Preclinical studies also show a clear role for the immune system in the manifestation of the abscopal effect. Demaria et al. [17] found that T cells mediate distant tumor inhibition caused by RT. Work on a mouse model of breast cancer showed a decrease in pulmonary metastases and improved survival only in mice treated with RT in combination with cytotoxic T-lymphocyte associated protein 4 (CTLA4) blockade.
Along with programmed cell death 1 ligand 1 (PD-L1) and programmed cell death 1 (PD-1), there is another inhibitory mechanism. Cytotoxic antigen, which associated with T lymphocytes (CTLA4), can compete with CD80/86 and inhibit T cell activation [18]. After that, PD1, which are expressed on the surface of T cells, bind to PD-L1 and block immune responses. As a result, T cells do not affect on the tumor and there is no objective response to treatment. Thus, tumor-associated immunosuppressive mechanisms play a key role in blocking the abscopal effect of radiation therapy.
In 2018, James Ellison (USA) and Tasuku Honjo (Japan) received the Nobel Prize for their revolutionary discovery in understanding the mechanism of cancer immunotherapy and the development of tumor immunotherapy. Both scientists in independent studies studied the same phenomenon, and found two different immune checkpoints (control points)ŌĆömechanisms for inhibiting T cell activity and suppressing the overall immune response [19]. T cells interact with dendritic cells and form a complex system of many proteins connecting to each other on membranes, or the immune synapse. Some of these protein molecules are co-stimulants and can contribute to enhanced activation of lymphocytes. The discovery of Ellison and Honjo concerned molecules of inhibitors of the T cell responseŌĆöCTLA-4 and PD-1. Drugs-inhibitors of immune checkpoints have made it possible to achieve certain successes in the immunotherapy of cancer patients. The checkpoint inhibitors CTLA1, PD1, and PDL is rapidly becoming a promising therapeutic area in RT, due to which it is proposed to increase antitumor immunity and increase the frequency of the abscopal effect [18]. The combination of radiation and immune therapy can potentially help overcome tumor-induced immunosuppression, which causes a lack of activating effect of RT on tumor-specific T cells. RT, damaging the DNA of tumor cells, leads to apoptosis, aging and cellular autophagy, induces immunogenic death of tumor cells. This is cross-priming of tumor-specific T cells, the generation of various inflammatory signals that contribute to the activation of dendritic cells [20]. Immunotherapy removes the block from the activation of tumor-specific T cells and leads to a significant antitumor effect and an abscopal effect (Fig. 1).
Clinical Cases
The abscopal effect was shown in many malignant neoplasms, such as renal cell carcinoma, melanoma, lymphoma, hepatocellular carcinoma and other types, but this is still a rare and poorly studied phenomenon [12].
As a result of the literature analysis, 35 clinical studies were found in the range from 1973 to 2019 (46 years), which describe 51 cases of the abscopal effect at various locations [21-54] (Tables 1, 2). It was found that in 33% (17/51 patients) of cases, the abscopal effect was recorded in melanoma. Also often the primary location of the tumor was the kidney (18%, 9/51 cases) and the lung (14%, 7/51 cases). The most common localizations of metastases in which the abscopal effect was recorded were the lung (41%), lymph nodes (31%), and the liver (15.7%). It is also worth noting that in only 13 out of 51 cases, multiple regression of tumor metastases was observed.
Nowadays, the literature data are very different regarding depending on the presence or absence of combined treatment (radiation + immunotherapy), so all studies were divided into two groups. The first group included cases with a combination of radiation and immunotherapy (27/51 studies) (Table 1). The second group consisted of patients only with RT (24/51 cases) (Table 2).
In 17 of 27 cases (63%) in patients of the first group, immunotherapy was carried out with a CTLA4 inhibitor drug, which leads to an increase in the T cell antitumor response, ipilimumab. The average time after which the abscopal effect was recorded is 3.3 months (from 1 to 13 months).
In patients of the second group, the abscopal effect was observed on average after 5.4 months (from 1 to 24 months) (Table 2). In 29% (7/24 cases) of patients of the second group there was kidney cancer as primary localization, most often the abscopal effect was detected with distant metastases in the lung and lymph nodes (41% and 32%, respectively).
After a comparative analysis of the group of patients with the presence of RT alone and the group with combined treatment, the number of cases at the beginning of the 20th century significantly increased with the advent of immunotherapy. Twenty-seven cases were recruited in 13 years, while only with RT 24 cases were recruited in 46 years. Also, the time of manifestation of the abscopal effect with regression of distant metastases was reduced from 5.4 to 3.3 months. The differences here are statistically insignificant (p = 0.141, by StudentŌĆÖs criterion), but this can be attributed to the small number of samples and the heterogeneity of the drugs used for immunotherapy. Control point inhibitors and CTLA4 give a more frequent abscopal effect (on average after 2.5 months), then other immunotherapy methods.
Discussion and Conclusion
Despite the long history of the study, the abscopal effect is still a rare and poorly studied phenomenon. However, with the advent of new approaches to immunotherapy, which allows overcoming the tumor associated immunosuppression in combination with RT, the frequency of registration of cases of abscopal effect began to increase significantly. The same conclusion was reached by a number of authors that after the start of using agents on CTLA-4 or the PD-1/PD-L1 axis, the number of registered patients with an abscopal effect increased [55,56]. The study of the molecular mechanisms of the RT-induced antitumor immune response is becoming a relevant topic. There is an opportunity to identify predictive markers that allow you to consciously use the combined treatment with the induction of an abscopal effect more consciously. Also identify new targets for drug exposure in order to induce an abscopal effect.