 |
 |
AbstractMucoepidermoid carcinoma (MEC) is the most common malignancy of minor salivary glands in adults. Pulmonary MEC is extremely uncommon, comprising only 0.1‚Äď0.2% of the primary lung malignancies and <1% of primary bronchial tumors. It is even rarer in children, and literature is limited to a few case reports only. Here we present a case report of a 9-year-old boy diagnosed with primary MEC of the trachea along with a review of the literature. A 9-year-old male child presented with complaint of dry cough for two years which was later associated with shortness of breath after one year. Bronchoscopic examination revealed a growth arising from right lateral wall of carina occluding 50% of the lumen and detailed histopathological examination revealed it to be a MEC of the trachea. The patient underwent local excision of the tumor with primary anastomosis. Because of positive margin, adjuvant radiotherapy of 60 Gy in 30 fractions was given to the tumor bed. The patient tolerated the treatment well and is disease free at 6 months follow-up. Experience with MEC of the trachea in children is limited, and optimal treatment protocols have not been defined, with current treatment mainly extrapolated from MEC of the salivary glands.
IntroductionPrimary malignancies of the lung are very rare in the pediatric population, accounting for <1% of all pediatric malignancies [1]. Even rarer are the primary malignancies of the trachea-bronchial tree in this population. Mucoepidermoid carcinoma (MEC) is uncommon in lungs constituting only 0.1%‚Äď0.2% of the primary lung malignancies [2]. In the pediatric age group, it represents 9%‚Äď10% of all malignant primary lung tumors [1]. MEC is mainly the tumor of salivary glands and was initially described by Smetana et al. [3] in 1952. Pediatric cases of MEC of the trachea are mainly limited to fewer than 10 cases reported in literature so far and are often clubbed with MEC of the whole tracheobronchial tree.
Here, we report a case of 9-year-old male with MEC of the trachea treated with surgery and postoperative radiotherapy. Till now, only fewer than ten cases of primary MEC of trachea has been reported in children. Rarity of the disease may often lead to delayed diagnosis or misdiagnosis.
Case ReportA 9-year-old male child presented with complaint of dry cough for 2 years which was later associated with shortness of breath after 1 year. There was no seasonal variation. In view of overlapping specific symptoms patient was earlier diagnosed as case of asthma and took treatment for the same but to no relief [4].
A bronchoscopy was done which revealed growth from right lateral wall of carina occluding 50% of the lumen. Positron emission tomography (PET) scan showed a heterogeneous enhancing non fluorodeoxyglucose (FDG) avid lesion in right lateral wall of trachea 2.5 cm above the carina. Patient underwent excision of the mass and primary anastomosis. On pathological examination of the specimen, grossly the tumor showed both exophytic and endophytic growth which was extending and eroding tracheal ring cartilage.
Microscopically (Fig. 1A‚Äď1F) it revealed ill-defined tumor within the sub-epithelium giving polypoidal appearance to overlying mucosa. It seemed to be arising from the submucosal glands and eroding hyaline cartilage at places. Tumor cells were predominantly composed of intermediate cells and were arranged in the form of nests, islands, trabeculae and at places solid sheets. Mitosis was infrequent. There were no areas showing cystic degeneration, significant nuclear atypia or necrosis. Circumferential resection margin was close and distal and proximal resection margins were involved by the tumor. In consideration of the above findings final diagnosis was of tracheal MEC of intermediate grade.
In view of positive margins he was given adjuvant radiotherapy with a dose of 60 Gy in 30 fractions in 6 weeks with volumetric modulated arc therapy (VMAT) on a 6-MV linear accelerator. The VMAT was planned using two arcs. A 95% of the prescribed dose was delivered to 98% of the PTV. The dose constraints to the organs-at-risk were well respected. The Dmax of the spinal cord was 44 Gy. V5 of B/L lungs was 25.6%, V20 was 13.3% and V30 was 4.7%. Dmean received by heart was 0.89 Gy. Esophagus received a Dmean of 22 Gy and Dmean of thyroid gland was 45 Gy.
During the course of radiotherapy, the patient developed grade 1 skin reactions and grade 2 esophagitis which were managed conservatively without requiring any treatment gap. The patient tolerated the treatment well and is presently asymptomatic and is disease free both clinically and radiologically at 6 months of follow-up. Dose distribution of the final plan of radiotherapy is shown in Fig. 2.
DiscussionMECs of tracheobronchial tree is extremely rare neoplasm in pediatric population. MEC represents 0.2% of all lung tumors [2,5]. So far fewer than ten patients have been reported in literature in children less than 10 years of age. Table 1 summarizes the cases of MEC of trachea in children less than 10 years of age. All the patients mentioned in the table were managed with surgical resection and none of them received any adjuvant treatment. MEC of the lung presents in wide range of age groups however, younger patients are rare. In a cohort of 18 patients age range from 29-86 years was reported by Rapidis et al. [6]. Ozawa et al. [7] reported age range of 22‚Äď86 years in a cohort of 43 patients and an age range of 33‚Äď70 years in a cohort of 30 patients. Reports in involving pediatric age group of less than 10 years is very rare.
Due to its rarity and overlapping symptoms with bronchial asthma the diagnosis is often delayed in a child presenting with recurrent upper respiratory tract symptoms. Diagnosis delay for up to 20 months has been reported in literature. Children with MEC of the tracheobronchial tree presents most commonly with coughing, wheezing, bronchitis, fever, chest pain and dyspnoea due later stage of the disease. Coughing, haemoptysis, fever, wheezing and recurrent pneumonia were the most common signs in patients in studies by Tsuchiya et al. [8] and Dinopoulos et al. [9]. In the present report the patient developed dry cough for about 2 years duration which was later accompanied by shortness of breath due to tracheal luminal compromise. During the course the patient was initially a diagnosed as a case of bronchial asthma and thus there was a diagnosis delay of approximately 2 years.
The investigation often begins with chest X-ray and CT-scan of the chest. The tumors usually presents as a lobulated exophytic luminal mass. Bronchoscopy is indicated for better visualisation of the tumors and to take biopsy for confirmatory diagnosis.
MEC of the tracheobronchial tree is histologically similar to MEC of salivary glands and these are categorised into low-, intermediate-, and high-grade tumors based on nuclear pleomorphism, necrosis, type of cell (mucous, intermediate, and epidermoid), and degree of mitotic activity [10]. Low-grade tumors are slow growing and are generally managed by surgery alone whereas high-grade tumors have poor prognosis due to greater chance of recurrences and metastasis and often require multimodality treatment [10]. In a study by Heitmiller et al. [11] on 18 patients, patients with high-grade tumors did much worse with all of them dying within one and half years. However, all patients with low-grade tumors were reportedly alive at 4.7 years.
MEC of the tracheobronchial tree in children should be considered potentially malignant. However due to slow growing nature of these tumors a prompt diagnosis and early surgical treatment is necessary. Complete surgical resection with en-bloc removal of tracheal rings and reconstruction of the trachea is the primary treatment [12-16]. Long-term cure has been achieved with complete resection in low grade MEC patients in most of the studies. However, due to scarcity of the literature available, the role of adjuvant treatment is still unclear especially in intermediate grade histology.
MECs of the salivary glands have been treated successfully with radiotherapy in the adjuvant setting. Studies have shown benefit of postoperative radiotherapy in patients with positive surgical margins, high-grade histology and recurrences in MECs of salivary glands [6]. Adequate local control in salivary gland MECs with positive margins have been achieved with dose of 55 Gy or more of adjuvant radiotherapy [17]. With the scarcity of literature available for adjuvant treatment in tracheobronchial MECs and extrapolating the role of radiotherapy in salivary gland MECs, adjuvant radiotherapy with a dose of 60 Gy was delivered to the patient in our report. Adjuvant treatment have been used in cases of R2 resection or in case of high-grade tumors or a recurrence. Fauroux et al. [18] reported a child who had recurrence after 3 years of initial resection. Benefit of combined chemotherapy and radiotherapy has been shown in few studies in high grade MEC and in recurrences. Risk/benefit ratio of adjuvant treatment should be explained to patients and guardians. In the index case, as the mean dose to thyroid gland was 45 Gy, parents have been counselled regarding the potential risk of hypothyroidism in long term and frequent monitoring with thyroid function test. Patient should also be counselled and monitored for any possible risk of radiation induced cancers especially in pediatric population.
There have been few reports studying the role of targeted therapy in treatment of MEC of tracheobronchial tree. MECs of the salivary gland are known to frequently overexpress epidermal growth factor receptor (EGFR). Specimens of pulmonary origin MECs were also tested for EGFR mutations by Han et al. [19], EGFR overexpression has been reported in few cases of high-grade MEC. Tyrosine kinase inhibitors (TKIs) are a known treatment option in adjuvant setting in EGFR mutation positive non-small-cell lung cancer. Partial response has been seen in some of the patients with high grade MECs with the use of EGFR tyrosine kinase [19]. Prospective randomized studies are needed to further investigate the role of EGFR TKIs in tracheal MEC.
Childhood MECs of the tracheobronchial tree tend to do better compared to those in adults. In a study of Chin et al. [10], 1-year survival of only 20% in high-grade MEC in adult patients and 80% for low- and intermediate-grade MEC was found. However, in paediatric patients of MEC the 5-year and 10-year overall survival and disease-free survival was found to be 100% in study done by Neville et al. [20].
MEC of the tracheobronchial tree is extremely rare in pediatric and is often overlooked while considering in the differential diagnosis for patients with signs of upper respiratory tract obstruction. Complete surgical resection is the primary treatment modality for low-grade MEC. However, high-grade and recurrent MECs of tracheobronchial tree in adults have been managed with radiotherapy and chemotherapy in the adjuvant setting [18]. Role of radiotherapy is also important in tracheal tumors where symptomatic control from luminal compromise by the tumour is required. Prospective studies are required for better defining treatment strategies of this malignancy.
NotesStatement of Ethics Written informed consent was obtained from the parents and ethical clearance was exempted by institutes ethics committee. Author Contribution Conceptualization, RM. Investigation and methodology, DKU. Project administration, RM, DKU. Supervision, RM. Writing of the original draft, DKU. Writing of the review and editing, RM, NJP, AB, SG, NB, DK. Formal analysis, RM, DKU. Data curation, DKU, RM. All the authors have proofread the final version. Fig. 1.(A) Squamous cells (black arrow, large cells having round pyknotic nuclei and abundant cytoplasm) and intermediate cells (blue arrow, small cells with high nuclear to cytoplasmic ratio and less cytoplasm). (B) Intermediate cells (blue arrow). (C) Mucus/vacuolated cells (red arrow, cells with abundant intracytoplasmic mucinous vacuoles) and respiratory lining (black arrow). (D) Higher power of previous image to appreciate mucus/vacuolated cells. (E) Periodic Acid-Schiff (PAS) stain and Alcian blue stain highlighting the intracytoplasmic mucin in the mucus/vacuolated cells in blue color (black arrow). (F) P63 nuclear positivity in squamous and intermediate cells. 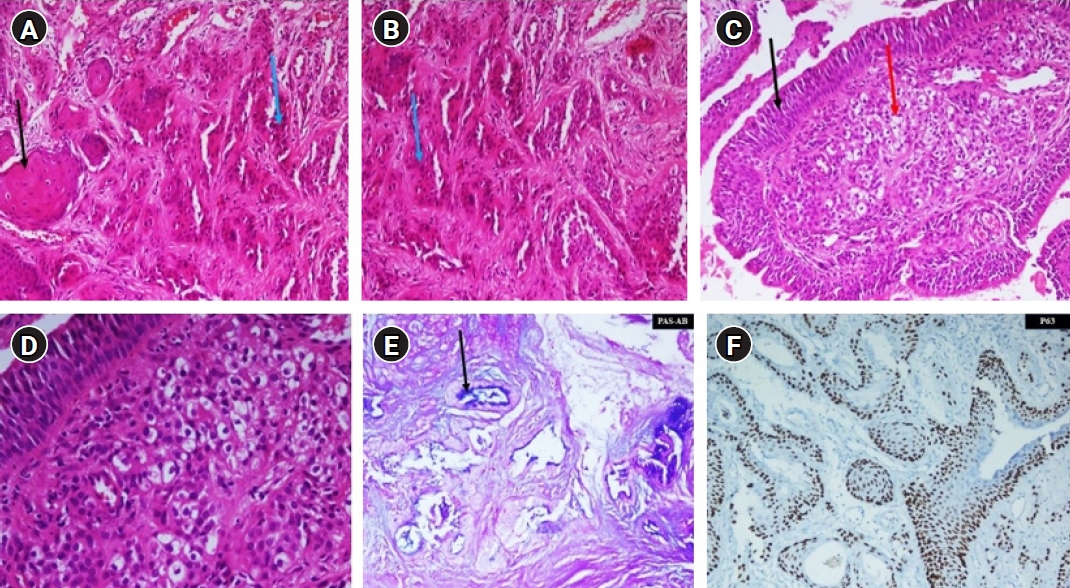
Table 1.Summary of the cases of mucoepidermoid carcinoma trachea
References1. Dishop MK, Kuruvilla S. Primary and metastatic lung tumors in the pediatric population: a review and 25-year experience at a large children‚Äôs hospital. Arch Pathol Lab Med 2008;132:1079‚Äď103.
3. Smetana HF, Iverson L, Swan LL. Bronchogenic carcinoma; an analysis of 100 autopsy cases. Mil Surg 1952;111:335‚Äď51.
4. Lin CH, Chao YH, Wu KH, Lin WC. Primary mucoepidermoid carcinoma at the carina of trachea presenting with wheezing in an asthmatic child mimicking an attack of asthma: a case report. Medicine (Baltimore) 2016;95:e5292.
5. Papiashvilli M, Ater D, Mandelberg A, Sasson L. Primary mucoepidermoid carcinoma of the trachea in a child. Interact Cardiovasc Thorac Surg 2012;15:311‚Äď2.
6. Rapidis AD, Givalos N, Gakiopoulou H, et al. Mucoepidermoid carcinoma of the salivary glands: review of the literature and clinicopathological analysis of 18 patients. Oral Oncol 2007;43:130‚Äď6.
7. Ozawa H, Tomita T, Sakamoto K, et al. Mucoepidermoid carcinoma of the head and neck: clinical analysis of 43 patients. Jpn J Clin Oncol 2008;38:414‚Äď8.
8. Tsuchiya H, Nagashima K, Ohashi S, Takase Y. Childhood bronchial mucoepidermoid tumors. J Pediatr Surg 1997;32:106‚Äď9.
9. Dinopoulos A, Lagona E, Stinios I, Konstadinidou A, Kattamis C. Mucoepidermoid carcinoma of the bronchus. Pediatr Hematol Oncol 2000;17:401‚Äď8.
10. Chin CH, Huang CC, Lin MC, Chao TY, Liu SF. Prognostic factors of tracheobronchial mucoepidermoid carcinoma: 15 years experience. Respirology 2008;13:275‚Äď80.
11. Heitmiller RF, Mathisen DJ, Ferry JA, Mark EJ, Grillo HC. Mucoepidermoid lung tumors. Ann Thorac Surg 1989;47:394‚Äď9.
12. Romao RL, de Barros F, Maksoud Filho JG, et al. Malignant tumor of the trachea in children: diagnostic pitfalls and surgical management. J Pediatr Surg 2009;44:e1‚Äď4.
13. Chan EY, MacCormick JA, Rubin S, Nizalik E. Mucoepidermoid carcinoma of the trachea in a 4-year-old boy. J Otolaryngol 2005;34:235‚Äď8.
14. Desai DP, Mahoney EM, Miller RP, Holinger LD. Mucoepidermoid carcinoma of the trachea in a child. Int J Pediatr Otorhinolaryngol 1998;45:259‚Äď63.
15. Noda S, Sundaresan S, Mendeloff EN. Tracheal mucoepidermoid carcinoma in a 7-year-old child. Ann Thorac Surg 1998;66:928‚Äď9.
16. Kim J, Park C, Kim K, et al. Surgical resection of mucoepidermoid carcinoma at the carina in a 9-year-old boy. J Pediatr Surg 1998;33:1561‚Äď2.
17. Hosokawa Y, Shirato H, Kagei K, et al. Role of radiotherapy for mucoepidermoid carcinoma of salivary gland. Oral Oncol 1999;35:105‚Äď11.
18. Fauroux B, Aynie V, Larroquet M, et al. Carcinoid and mucoepidermoid bronchial tumours in children. Eur J Pediatr 2005;164:748‚Äď52.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |