 |
 |
AbstractPurposeThe target delineation of whole ventricle radiotherapy (WVRT) in germinoma varies among radiation oncologists, especially regarding the inclusion of the prepontine cistern (PC). We evaluated the outcome of PC-sparing WVRT in localized germinoma.
Materials and MethodsWe identified 87 localized intracranial germinoma patients who received radiotherapy (RT) following chemotherapy between 1999 and 2020. By institutional policy, RT for localized germinoma excluded PC from the target volume. WVRT was delivered to 65 patients (74.7%) and involved field radiotherapy (IFRT) to 22 patients (25.3%). The median dose was 45.0 Gy (range, 23.4 to 55.8 Gy) for the primary tumor and 19.8 Gy (range, 14.4 to 36.0 Gy) for the whole ventricle. We analyzed the dosimetric differences of the organs at risk between the PC-excluding plans and the PC-including ones.
ResultsThe median follow-up duration was 7.8 years (range, 1.0 to 22.5 years). The 10-year recurrence-free survival and overall survival rates were 86.3% and 90.9%, respectively. The recurrences occurred in eight patients (8.7%), including five patients after IFRT and three after WVRT. Five of them showed recurrences at lateral ventricles and only one patient experienced spinal cord relapse. However, no relapse in the PC occurred. Endoscopic third ventriculostomy was not a significant prognostic factor. The dosimetric comparisons showed significantly lower mean doses to the brainstem and the cochleae when the PC was excluded.
IntroductionIntracranial germinoma is an uncommon brain tumor in midline structures, mainly affecting children and young adults. It has a favorable prognosis with a 10-year overall survival around 95% [1,2]. Intracranial germinoma is characterized by a propensity for cerebrospinal fluid (CSF) spread and high radiosensitivity. The main principle of the treatment strategy has been de-intensification to minimize long-term toxicity by reducing radiation volume or dose. For localized intracranial germinoma, whole ventricle radiotherapy (WVRT) became a well-established standard treatment, replacing craniospinal irradiation (CSI) and whole brain radiotherapy (WBRT) [3,4].
Despite a consensus on treatment strategy, the definition of whole ventricle (WV) volume has not reached a robust consensus among clinicians or in guidelines, especially regarding the prepontine cistern (PC), a CSF subarachnoid space ventral to the pons and dorsal to the clivus. The survey of Mailhot et al. [5] revealed the most significant discrepancy in the inclusion of the PC among pediatric radiation oncologists. Over half of the responders (53.5%) stated that the PC would not be included, while 32.6% said that they would include it only after the third ventriculostomy. Consequently, the Children’s Oncology Group (COG) generated a WVRT atlas for clinical trials in which, the inclusion of the PC was optional for according to clinician’s preference [6]. However, this can lead to a significant deviation in clinical practice and prospective protocols, which can affect the resulting analysis.
In accordance with our institutional policy, radiotherapy for localized germinoma excludes the PC from the target volume, on the assumption that there is a low risk of recurrence related to the PC. In this study, we evaluated the oncological safety and outcome of WVRT excluding the PC in localized germinoma.
Materials and Methods1. PatientsThe Seoul National University Hospital Institutional Review Board approved this study and waived the requirement for patient informed consent (IRB No. H-2204-141-1319). Since the introduction of chemotherapy in the management of germinoma in 1999, chemotherapy followed by radiotherapy, has become our treatment protocol. Therefore, we reviewed the medical records of localized germinoma patients who received upfront chemotherapy followed by WVRT or involved field radiotherapy (IFRT) from January 1999 to December 2020. We excluded patients with CSF seeding (positive craniospinal fluid cytology or positive imaging) or multifocal lesions (more than two lesions) at the time of diagnosis. The patients with serum alpha-fetoprotein (AFP) above 10 ng/mL or serum beta-human chorionic gonadotropin (B-HCG) above 50 mIU/mL were also excluded. A total of 87 patients were eligible for the analysis.
Table 1 summarizes the baseline characteristics of the patients. The median age was 15 years (range, 4 to 44 years), and 72 patients (82.8%) were male. The Eastern Cooperative Oncology Group (ECOG) performance status in 79 patients (90.8%) was between 0 and 1. The solitary disease was found in 72 patients, and thereof, the most common solitary lesion was the pineal gland (n = 39) followed by the suprasellar (n = 16). We defined bifocal tumor as a non-solitary disease originating from two different intracranial sites. Of 15 patients with bifocal germinomas, three patients had tumors occurring other than in the suprasellar or pineal glands; these were in the thalamus and brain stem, both basal ganglia, and quadrigeminal cistern and tectal midbrain, respectively. Histologic confirmation was undertaken by stereotactic biopsy in 26 patients, endoscopic biopsy in 44, open biopsy in nine, and tumor removal in eight (subtotal removal in five, near total removal in two, and gross total removal in one). Endoscopic third ventriculostomy (ETV) was performed on 31 patients (35.6%). Information on the initial tumor markers was available for all but three patients. Eleven patients had serum B-HCG levels ranging from 6 to 28 mIU/mL, and three patients had serum AFP levels ranging from 9 to 10 ng/mL.
2. TreatmentSince 1992, our institution has had a combined treatment policy of chemotherapy followed by radiotherapy. Accordingly, the patients in our study were treated with the following chemotherapy regimens: two cycles (range, 1 to 4 cycles) of bleomycin, etoposide, and cisplatin or etoposide and carboplatin for 36 patients; four cycles of etoposide, carboplatin, and cyclophosphamide for 30 patients; four cycles of bleomycin, etoposide, cisplatin, cyclophosphamide for two patients; two cycles of cisplatin, etoposide, ifosfamide (VIP) for six patients; five cycles (range, 4 to 5 cycles) of cisplatin, etoposide, cyclophosphamide, and vincristine (CCG 9921A or 9931A) for seven patients; and “8-in-1” (solumedrol, vincristine, lomustine, procarbazine, hydroxyurea, cisplatin, cytosine arabinoside, and cyclophosphamide) for four patients. After upfront chemotherapy, 53 patients achieved a complete response (CR), 29 patients achieved a partial response, and five remained stable based on an MRI study before radiotherapy (Table 1).
Previously, as our main treatment, IFRT was delivered to the tumor bed with a 2-cm margin mainly using a 2D technique. For WVRT, contrast-enhanced T1/T2-weighted magnetic resonance imaging (MRI) was fused with simulation CT images. Gross tumor volume (GTV) was pre- or post-chemotherapy tumor bed or operative bed based on T1-enhanced and T2 high FLAIR MRI images. Clinical target volume (CTV) was expanded 5–10 mm from GTV. In the case of disease arising from the basal ganglia or thalamus, a 15-mm margin was added to the tumor bed, including T2 high signal intensity. Based on the T2-weighted MRI, the WV treatment volume encompassed the lateral, third, fourth ventricles, the suprasellar cistern, and the pineal cistern according to the COG guideline [6]. The PC was excluded from the WV volume in all patients (Fig. 1A). The large field included the primary tumor bed as well as the WV. Additional doses to the tumor bed were delivered sequentially.
Table 2 presents the radiation treatment characteristics. WVRT was delivered to 65 patients (74.7%) and IFRT to 22 patients (25.3%). The median total dose to the primary tumor bed was 45 Gy (range, 36 to 54 Gy) for IFRT and 45 Gy (range, 23.4 to 55.8 Gy) for WVRT. Patients who showed a CR to initial chemotherapy received the median dose of 45 Gy (range, 23.4 to 55.8 Gy) to the primary tumor bed, while those who did not show a CR were given the median dose of 50.4 Gy (range, 36.0 to 55.8 Gy). The WV was irradiated with the median dose of 19 Gy (range, 14.4 to 36.0 Gy).
3. Dosimteric analysisOf 65 WVRT patients, the radiation therapy plans for 33 patients were retrievable. The intensity-modulated radiotherapy (IMRT) technique was used in 30 cases, while three cases were planned using three-dimensional conformal radiotherapy (3D-CRT). To evaluate the dosimetric effect of excluding the PC, we additionally generated the new plans that included the PC of 33 patients (Fig. 1B). The corresponding treatment modality (IMRT or 3D-CRT) to the one utilized in the original plan was used. The brainstem and each cochlea were manually contoured according to the guideline [7]. We collected the dosimetric information from the original plans and the newly constructed ones.
4. Statistical analysisWe conducted statistical analysis by using Stata/SE 17 (StataCorp LLC, College Station, TX, USA). Overall survival (OS) was the duration from the date of initial histologic diagnosis to the date of the last visit or death from any cause. Similarly, recurrence-free survival (RFS) was calculated as the duration between the initial confirmation and any recurrence or death. We used the Kaplan-Meier method for survival analysis and a log-rank test to identify prognostic factors. Paired t-test was applied to compare the dose-volume metrics of the normal organ structures between the PC excluding plans and the PC including plans. The prescribed dose to 95% of the large field consisting of the WV and tumor bed was used as the reference.
Results1. Failure patternDuring the median follow-up of 7.8 years (range, 0.49 to 22.5 years), eight patients experienced recurrences, including five in IFRT and three in WVRT (Table 3). The median time to recurrence after radiotherapy was 2.3 years. Four of five recurrent patients after IFRT experienced relapses at the ventricular area. All of the recurrences in IFRT were out-field, except one case of in-field relapse at the pineal gland. For WVRT, there was one patient who demonstrated spinal cord relapse at L1–L4. Other two WVRT patients developed very late infield-relapses. After 10.6 years, relapses in one patient with WVRT occurred at the bilateral lateral ventricular walls as well as the primary tumor bed at the basal ganglia, extending to the ipsilateral frontal lobe and parietal lobe. Another patient with WVRT developed a new lesion near the fourth ventricle after 8.3 years. However, no recurrence in the PC was observed among the patients with recurrences.
2. Treatment outcomesAfter completion of radiotherapy, 32 of 34 non-CR patients eventually reached CR. Except them, there was one patient whose residual disease remained stable until the last follow-up. Another non-CR patient eventually experienced the disease relapse (patient #8 in Table 3). Overall, the 10-year RFS rate reached 86.3% (Fig. 2A) and the 10-year OS rate was 90.9% (Fig. 2B). When stratified by ETV, there was no significant difference in the 10-year RFS (85.8% vs. 87.2%; p=0.818) (Fig. 3A) and the 10-year OS rate (89.8% vs 93.2%; p=0.674) (Fig. 3B). Table 4 presents the results of the univariate and multivariate analysis, and none of the factors, including ETV, was significantly associated with RFS or OS.
All of the recurred patients received salvage treatment. Chemotherapy was given to all patients, while salvage radiotherapy was delivered as CSI to five patients and stereotactic radiosurgery (SRS) to one patient. One patient underwent total resection as initial treatment. Peripheral blood stem cell transplantation was also performed in two patients. Four patients (50.0%) were rescued after salvage treatment and remained alive until the last follow-up.
Of the eight deaths, two were attributed to the progression of recurrent disease. Bleomycin-induced pneumonitis and chemotherapy-induced neutropenic sepsis accounted for a further two; one patient with WVRT died of secondary malignancy; and the cause of death for the remaining three was either unrelated to the disease or unknown.
3. Dosimetric analysis
Table 5 presents the dosimetric comparison of the organs at risk between the PC-excluding plans and the PC-including plans among 33 WVRT patients. The dose parameters were measured for three normal structures: the brainstem, the left cochlea, and the right cochlea. Their original plans were compared with the newly generated plans which included the PC. Excluding the PC significantly reduced the Dmean (Gy) and the Dmean (%) for the brainstem (16.9 Gy vs. 20.3 Gy, p < 0.001 and 81.5% vs. 97.5%, p < 0.001, respectively). Similarly, the mean dose for the left and the right cochlea was significantly lower in the PC-excluding plans (8.3 Gy vs. 15.6 Gy, p < 0.001 and 9.7 Gy vs. 15.8 Gy, p < 0.001, respectively).
4. Secondary malignancyDuring the follow-up period, three patients developed secondary malignancy one in WVRT and two in IFRT. The median time to the onset of secondary cancer was 20.1 years, ranging between 9.0 and 21.0 years after radiotherapy. One patient developed medulloblastoma within the prior radiotherapy field 9 years after WVRT and eventually died of disease progression. Two other patients were diagnosed with meningioma located outside the previous treatment fields 20.1 and 20.0 years after IFRT, respectively. They underwent surgical resection, and one of them received adjuvant radiotherapy due to histologic grade 2 with brain invasion.
Discussion and ConclusionTo the best of our knowledge, this is the first study to report the outcome of excluding the PC from WVRT in localized germinoma after upfront chemotherapy. Since we adopted WVRT at our institution, the PC has been excluded from the WVRT volume. However, we did not observe any failure near the PC and achieved comparable disease control and OS corresponding with previous reports [1,8-12]. Besides, we confirmed significant dose reduction in the normal organ structures such as the brainstem. As the primary interest has shifted on the deintensification of the treatment to reduce long-term toxicity, reduced WVRT volume can be a reasonable and viable strategy.
The clinical outcomes of localized germinoma in our study were similar to the previous studies, but there were some differences regarding the treatment field. The 10-year RFS and OS were 86.3% and 90.9%. The 5-year OS rates for localized germinoma after chemoradiotherapy ranged from 88% to 97% and the 5-year DFS ranged between 95%–98.8%. In the SIOP CNS GCT 96 trial which showed the feasibility of replacing CSI alone by focal irradiation with chemotherapy, the rates of 5-year progression-free survival and OS were 88% and 96%, respectively [10]. Koh et al.’s multi-institutional Asian study [2] reported 97.4% of OS and 92.6% of RFS for non-metastatic germinoma with 19.8 Gy WVRT. One major difference between our study and others was the treatment field determined by tumor location, tumor multiplicity, and chemotherapy response. The SMC-G13 trial by Lee et al. [11] resulted in 5-year PFS of 96.7% and OS of 96.2% in localized germinoma patients. In their study, whole brain radiotherapy was given in case of basal ganglia germinoma, while all patients in our study received IFRT or WVRT regardless of tumor location. Byun et al.'s single institutional retrospective study [1] included 19 patients with solitary tumor treated by CSI after chemotherapy. In a Korean prospective multicenter cohort study by Lee at al. [9], the treatment field for solitary tumor was extended to CSI in case of non-CR after upfront chemotherapy. In our study, the boost dose was modified in response to chemotherapy, and patients received either IFRT or WVRT if the tumor was solitary or bifocal without evidence of metastasis.
Despite limited data regarding the incidence of recurrence in the PC, the risk of failure in the PC does not seem high based on the treatment outcomes after IFRT. The SFOP study revealed that most failures occurred near brain parenchyma such as the frontal lobe, temporal lobe, occipital lobe and third ventricles after IFRT [13]. In the SIOP GCT trial by Calaminus et al. [10], localized germinoma patients with IFRT experienced ventricular relapses outside the radiation field. All the four ventricular relapses after IFRT were located within lateral ventricles in the study by Nakumara et al. [14]. Furthermore, no recurrence in the PC was observed even when radiotherapy was replaced by intensive chemotherapy in da Sailva et al.’s international CNS germ cell tumor study [15]. Consistent with other reports, ventricular relapse sites were mainly located near lateral ventricles in our study.
One of the considerations as to include the PC in treatment volume is the receipt of ETV which is usually recommended for pineal germinoma causing hydrocephalus [4]. It fenestrates the floor of the third ventricle, creating CSF flow from the third ventricle to the PC. This flow may also raise a concern for tumor cell spread to the PC. In our study, only one patient with ETV experienced isolated spinal cord relapse after WVRT. ETV was neither significantly associated with the risk of recurrence nor survival. Similarly, the COG ACNS1124 trial, a prospective study of WVRT dose reduction, also showed ETV did not carry an increased risk of recurrence in localized germinoma patients treated with a reduced dose after chemotherapy [16,17]. Among a total of eight relapses, two patients (25%) experienced parenchymal recurrences within the ETV surgical tract rather than ventricular area. Although including the surgical tract in the target volume will be an area for investigation, our finding and the ACNS1124 trial at least seem to suggest that excluding the PC area from the treatment field is not necessarily associated with disease control.
Excluding the PC from the treatment volume reduced radiation dose to the brainstem, potentially minimizing neurocognitive sequelae. Several previous studies reported the radiological evidence and clinical outcome of radiotherapy-induced brain damage. For example, the longitudinal imaging study of medulloblastoma patients found decline in fractional anisotropy within the brainstem after radiotherapy, suggesting axonal damage and demyelination [18]. Furthermore, dose-dependent white matter damage was detected by diffusion tensor imaging (DTI) and even at the low dose of 10–20 Gy, the DTI metrics which implied extracellular changes became significantly different at 9–11 months [19]. Such radiologic changes were also correlated with impaired cognitive and motor function in pediatric posterior fossa tumor survivals [20]. As these findings demonstrate the importance of minimizing radiation exposure as much as possible, we expect that reducing volume in WVRT would contribute to lowering the risk of radiation toxicity related to the brainstem such as cognition and motor function.
In addition to sparing the brainstem, excluding the PC also significantly spared the cochlea. In young and adolescent germinoma patients, the crude risk of radiotherapy-related ototoxicity is approximately 10%, with the risk of ototoxicity increasing particularly in those who received cisplatin, reaching a 10-year cumulative incidence of 39.2% [21]. The correlation between cochlear radiation dose and toxicity has been observed in pediatric brain tumor patients who underwent cranial radiation [22]. Although it was feasible to meet the recommended constraint for the cochlea (Dmean <35–45 Gy) in both the original plans and the PC including plans, it is still important to minimize the dose exposure as much as possible [7]. In a dosimetry analysis of the French Childhood Cancer Survivors Study (FCCSS), a correlation was found between inner ear dose and ototoxicity after cisplatin and radiation. Cohen-Cutler et al. [23] demonstrated that the minimal cochlear dose predicted hearing loss with an odds ratio of 1.64 per 10 Gy (p = 0.043). Although we did not have a detailed toxicity profile relevant to ototoxicity, excluding the PC in WVRT may not seem significant. However, given that cisplatin is one of the primary chemotherapy regimens for the treatment of germinoma, it is crucial to minimize the risk of developing ototoxicity by reducing radiation exposure to the cochlea, which is located very close to the PC.
Secondary cancer is a long-term complication of cancer survivors including germinoma. The incidence of secondary malignancy in germinoma patients ranges approximately between 5%–6% [24,25]. Previously, 5.3% of intracranial germinoma patients treated at our institution developed secondary malignancy with 20-year latent period (range, 4 to 26 years), which is consistent with the SEER database analysis of intracranial germ cell tumor patients [24,25]. One of the risk factors for secondary cancer development is the radiation field size [26]. Accordingly, we found a significantly higher risk of developing secondary malignancy after extended field radiotherapy such as WBRT or CSI compared to WVRT or IFRT [25]. Therefore, we expect that reducing the volume in WVRT would contribute to lowering the risk of radiation-related complications.
In an effort to reduce treatment volume, Yan et al. [27] from Princess Margaret Hospital explored the feasibility of excluding temporal ventricular horns from WVRT volume. Sparing temporal ventricular horns resulted in excellent disease control as well as a significantly reduced dose to the hippocampus and temporal lobes. Considering the correlation between hippocampus mean dose and hippocampal volume change in pediatric and young adult brain tumor patients, a reduced WVRT for these patients seems worthy of further exploration [28]. The study supported the safety of temporal horn sparing WVRT as they did not observe ventricular failure near the temporal horns among 29 patients with IFRT [1,29]. Although preserving the hippocampus is critical to neurocognitive outcomes, this approach may not always be viable. In our cohort, one patient experienced relapse at the temporal horn of the lateral ventricle, and further studies need to identify an eligible subset of germinoma patients.
Besides reducing WVRT volume, other strategies such as dose reduction and modern radiation techniques could help reduce treatment-related toxicity. The COG reported the favorable outcome of a reduced WVRT dose of 18 Gy in those who achieved a CR after chemotherapy. Despite a lack of statistical significance for non-inferiority, the 18 Gy WVRT regimen showed similar disease control with improved cognitive functioning compared with 24 Gy WVRT [17]. In addition, a study from the Hospital for Sick Children demonstrated the feasibility of omitting a boost dose to the tumor bed [30]. Advanced radiation techniques such as proton technique can be utilized as proton therapy offered dosimetric advantages for conformality and normal organ sparing compared to IMRT and volumetric modulated arc therapy for WVRT [31,32]. Proton beam radiotherapy was associated with a lower risk of secondary tumor development compared to IMRT (adjusted odds ratio = 0.31; 95% confidence interval, 0.26–0.36; p < 0.001) [33].
Our study, nevertheless, has some limitations. Firstly, we were unable to evaluate the functional consequences directly relevant to the brainstem and cochlea. This could be explained by the subclinical effect of radiation exposure or underestimation. Despite a long follow-up period, comprehensive neurological exams were not routinely performed, with primary emphasis being put on imaging studies and laboratory exams. Therefore, thorough neurological assessments related to specific structures will be valuable in understanding the toxicity profile after de-escalated WVRT. We nonetheless believe that it is still of paramount importance to minimize unnecessary radiation exposure, adhering to the principle of “As Low As Reasonably Achievable.” The overall risk of late toxicity would be less likely to occur than before with the use of modern radiotherapy techniques such as IMRT. Furthermore, chemotherapy regimens and radiation treatments were not consistent over the course of our study as the standard treatment for localized germinoma evolved over the period. However, the principle of radiation treatment was consistent as most of the patients were treated by one pediatric radiation oncologist.
In conclusion, our study showed the outcome of excluding the PC from WVRT in localized germinoma patients after chemotherapy, and our finding suggests that this can be one of the optimal strategies by reducing radiation treatment volume. However, future trials with long-term follow-up are required to reach a consensus on WVRT target volume.
NotesStatement of Ethics The Institutional Review Board of Seoul National University Hospital approved this study and waived the requirement for patient informed consent (IRB No. H-2204-141-1319). Fig. 1.Whole ventricular radiotherapy target delineation that shows (A) excluding prepontine cistern and (B) including prepontine cistern. 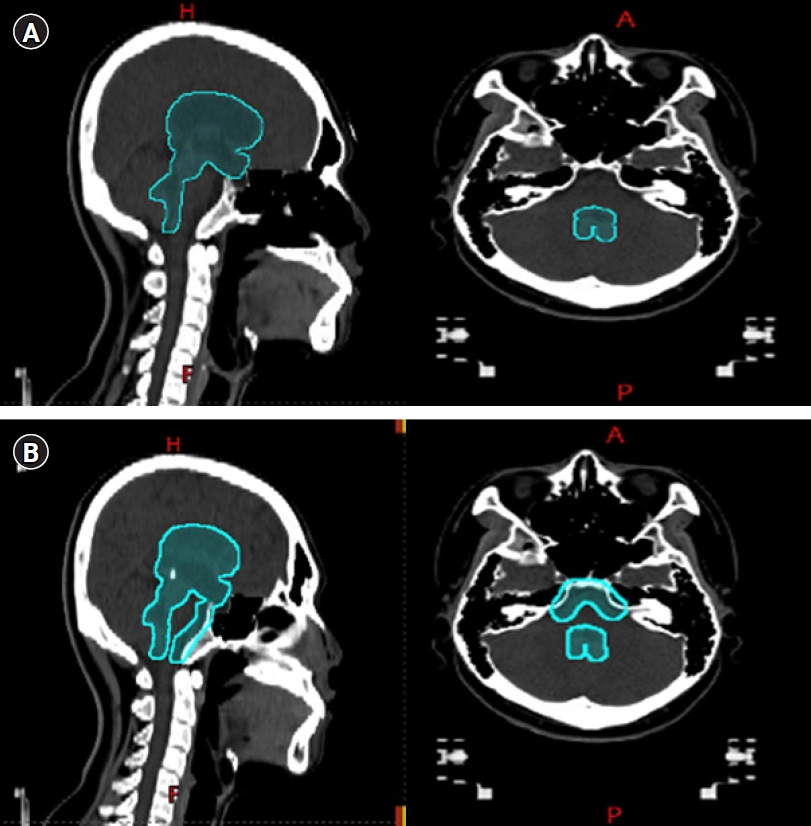
Fig. 2.Kaplan-Meier curves for recurrence-free survival (A) and overall survival (B) of patients with radiotherapy (RT). 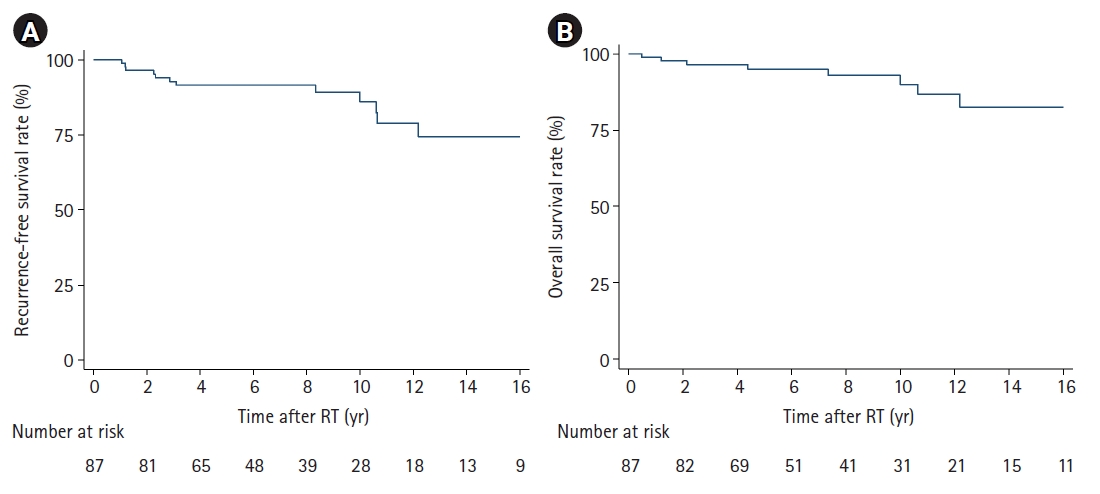
Fig. 3.Kaplan-Meier curves for recurrence-free survival (A) and overall survival (B) of patients with radiotherapy (RT) stratified by endoscopic third ventriculostomy (ETV). 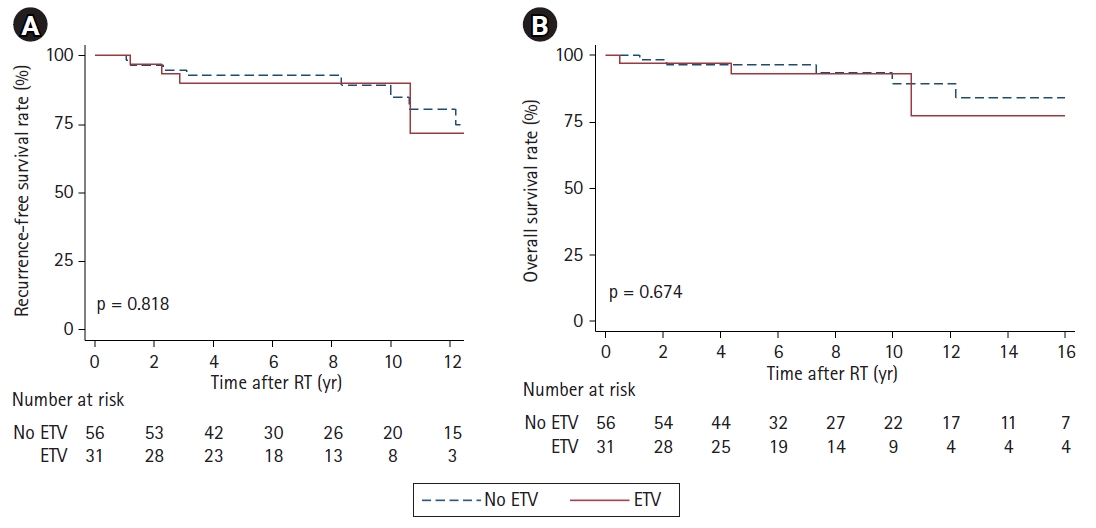
Table 1.Baseline characteristics of 87 localized intracranial germinoma patients
Table 2.Radiation treatment characteristics 87 localized intracranial germinoma patients
Table 3.Summary of the treatment outcomes for 8 patients with relapses
RT, radiotherapy; ETV, endoscopic third ventriculostomy; WVRT, whole ventricular radiotherapy; CTx, chemotherapy; CSRT, craniospinal radiotherapy; BG, basal ganglia; NED, no evidence of disease; IFRT, involved field radiotherapy; PBSCT, peripheral blood stem cell transplantation; SRS, stereotactic radiosurgery. Table 4.Univariate analysis of prognostic factors associated with RFS and OSa)
Table 5.Dosimetric comparison of the organs-at-risk between PC-excluding plans and PC-including plans among 33 patientsa)
References1. Byun HK, Yoon HI, Cho J, et al. Optimization of intracranial germinoma treatment: radiotherapy alone with reduced volume and dose. Int J Radiat Oncol Biol Phys 2020;108:657–66.
2. Koh KN, Wong RX, Lee DE, et al. Outcomes of intracranial germinoma-A retrospective multinational Asian study on effect of clinical presentation and differential treatment strategies. Neuro Oncol 2022;24:1389–99.
3. Nakamura H, Takami H, Yanagisawa T, et al. The Japan Society for Neuro-Oncology guideline on the diagnosis and treatment of central nervous system germ cell tumors. Neuro Oncol 2022;24:503–15.
4. Frappaz D, Dhall G, Murray MJ, et al. EANO, SNO and Euracan consensus review on the current management and future development of intracranial germ cell tumors in adolescents and young adults. Neuro Oncol 2022;24:516–27.
5. Mailhot R, Rotondo R, Murphy E, Caruso PA, Fullerton B, Tarbell NJ, et al. A consensus atlas for whole ventricular irradiation for pediatric germ cell tumors: survey results and guidelines. Int J Radiat Oncol Biol Phys 2012;84(3 Suppl):S65.
6. Children’s Oncology Group. ACNS1123 Atlas: whole ventricle target volume atlas for germ cell [Internet]. Monrovia, CA: Children’s Oncology Group; c2022 [cited 2023 Mar 17]. Available from: https://www.qarc.org/cog_protocol_resources.htm.
7. Scoccianti S, Detti B, Gadda D, et al. Organs at risk in the brain and their dose-constraints in adults and in children: a radiation oncologist's guide for delineation in everyday practice. Radiother Oncol 2015;114:230–8.
8. Eom KY, Kim IH, Park CI, et al. Upfront chemotherapy and involved-field radiotherapy results in more relapses than extended radiotherapy for intracranial germinomas: modification in radiotherapy volume might be needed. Int J Radiat Oncol Biol Phys 2008;71:667–71.
9. Lee DS, Lim DH, Kim IH, et al. Upfront chemotherapy followed by response adaptive radiotherapy for intracranial germinoma: prospective multicenter cohort study. Radiother Oncol 2019;138:180–6.
10. Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 2013;15:788–96.
11. Lee JW, Lim DH, Sung KW, et al. Induction chemotherapy reduces radiation therapy dose and volume in the treatment of intracranial germinoma: results of the SMC-G13 trial. Int J Radiat Oncol Biol Phys 2020;108:649–56.
12. Li B, Feng J, Chen L, et al. Relapse pattern and quality of life in patients with localized basal ganglia germinoma receiving focal radiotherapy, whole-brain radiotherapy, or craniospinal irradiation. Radiother Oncol 2021;158:90–6.
13. Alapetite C, Brisse H, Patte C, et al. Pattern of relapse and outcome of non-metastatic germinoma patients treated with chemotherapy and limited field radiation: the SFOP experience. Neuro Oncol 2010;12:1318–25.
14. Nakamura H, Takeshima H, Makino K, Kochi M, Ushio Y, Kuratsu J. Recurrent intracranial germinoma outside the initial radiation field: a single-institution study. Acta Oncol 2006;45:476–83.
15. da Silva NS, Cappellano AM, Diez B, et al. Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. Pediatr Blood Cancer 2010;54:377–83.
16. Yan R, Lin T, MacDonald S, et al. GCT-18: Endoscopic third ventriculostomy (ETV) and tumor biopsy are not associated with relapse rate or patterns in primary central nervous system (CNS) germ cell tumor (GCT). Neuro Oncol 2022;24(Supplement_1):i58.
17. Bartels U, Onar-Thomas A, Patel SK, et al. Phase II trial of response-based radiation therapy for patients with localized germinoma: a children's oncology group study. Neuro Oncol 2022;24:974–83.
18. Uh J, Merchant TE, Li Y, et al. Differences in brainstem fiber tract response to radiation: a longitudinal diffusion tensor imaging study. Int J Radiat Oncol Biol Phys 2013;86:292–7.
19. Connor M, Karunamuni R, McDonald C, et al. Dose-dependent white matter damage after brain radiotherapy. Radiother Oncol 2016;121:209–16.
20. Rueckriegel SM, Bruhn H, Thomale UW, Hernaiz Driever P. Cerebral white matter fractional anisotropy and tract volume as measured by MR imaging are associated with impaired cognitive and motor function in pediatric posterior fossa tumor survivors. Pediatr Blood Cancer 2015;62:1252–8.
21. Wong J, Goddard K, Laperriere N, et al. Long term toxicity of intracranial germ cell tumor treatment in adolescents and young adults. J Neurooncol 2020;149:523–32.
22. Bass JK, Hua CH, Huang J, et al. Hearing loss in patients who received cranial radiation therapy for childhood cancer. J Clin Oncol 2016;34:1248–55.
23. Cohen-Cutler S, Wong K, Mena V, et al. Hearing loss risk in pediatric patients treated with cranial irradiation and cisplatin-based chemotherapy. Int J Radiat Oncol Biol Phys 2021;110:1488–95.
24. Acharya S, DeWees T, Shinohara ET, Perkins SM. Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol 2015;17:741–6.
25. Lee JH, Eom KY, Phi JH, et al. Long-term outcomes and sequelae analysis of intracranial germinoma: need to reduce the extended-field radiotherapy volume and dose to minimize late sequelae. Cancer Res Treat 2021;53:983–90.
26. Kamran SC, Berrington de Gonzalez A, Ng A, Haas-Kogan D, Viswanathan AN. Therapeutic radiation and the potential risk of second malignancies. Cancer 2016;122:1809–21.
27. Yan M, Laperriere N, Velec M, et al. Redefining ventricular target volume in germinoma: is inclusion of temporal horns necessary? Int J Radiat Oncol Biol Phys 2019;104:852–8.
28. Hall M, Odia Y, Von Werne K, et al. RONC-13: change in hippocampus volume as a function of radiation dose: results from a prospective trial with standardized imaging and morphometric evaluation. Neuro Oncol 2022;24(Supplement_1):i179.
29. Byun HK, Yoon HI, Suh CO. Comment: In regard to Yan et al. Int J Radiat Oncol Biol Phys 2020;106:218–9.
30. Cheng S, Kilday JP, Laperriere N, et al. Outcomes of children with central nervous system germinoma treated with multi-agent chemotherapy followed by reduced radiation. J Neurooncol 2016;127:173–80.
31. Correia D, Terribilini D, Zepter S, et al. Whole-ventricular irradiation for intracranial germ cell tumors: Dosimetric comparison of pencil beam scanned protons, intensity-modulated radiotherapy and volumetric-modulated arc therapy. Clin Transl Radiat Oncol 2019;15:53–61.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

 |
 |