Clinical outcomes of stereotactic body radiotherapy for spinal metastases from hepatocellular carcinoma
Article information
Abstract
Purpose
To investigate the outcomes of patients with spinal metastases from hepatocellular carcinoma (HCC), who were treated by stereotactic body radiotherapy (SBRT).
Materials and Methods
This retrospective study evaluated 23 patients who underwent SBRT from October 2008 to August 2012 for 36 spinal metastases from HCC. SBRT consisted of approximately 2 fractionation schedules, which were 18 to 40 Gy in 1 to 4 fractions for group A lesions (n = 15) and 50 Gy in 10 fractions for group B lesions (n = 21).
Results
The median follow-up period was 7 months (range, 2 to 16 months). Seven patients developed grade 1 or 2 gastrointestinal toxicity, and one developed grade 2 leucopenia. Compression fractures occurred in association with 25% of the lesions, with a median time to fracture of 2 months. Pain relief occurred in 92.3% and 68.4% of group A and B lesions, respectively. Radiologic response (complete and partial response) occurred in 80.0% and 61.9% of group A and B lesions, respectively. The estimated 1-year spinal-tumor progression-free survival rate was 78.5%. The median overall survival period and 1-year overall survival rate were 9 months (range, 2 to 16 months) and 25.7%, respectively.
Conclusion
SBRT for spinal metastases from HCC is well tolerated and effective at providing pain relief and radiologic response. Because compression fractures develop at a high rate following SBRT for spinal metastases from primary HCC, careful follow up of the patient is required.
Introduction
Approximately 70% of patients with cancer have evidence of metastatic disease at the time of death [1]. The spinal column is the most common bony site for metastasis, occurring in 40% of patients with cancer [2]. Spinal cord compression from epidural metastasis occurs in 5% to 10% of patients, and in up to 40% of patients with pre-existing metastases to bones other than vertebrae. Of the patients with spinal metastasis, 10% to 20% develop symptomatic spinal cord compression [34].
Palliative radiotherapy (RT) is widely used as the treatment of choice for spinal metastases, whereas surgery is reserved for selected patients with spinal instability. Randomized studies comparing short-course and long-course RT have not found significant differences in response, functional outcome, or toxicity between the two types of RT [5]. However, long-course RT has provided longer duration of response. In the United States, a radiation scheme of 30 Gy in 10 fractions is considered the standard of care, and shorter fractionation schedules are typically reserved for those with relatively advanced disease. The previous randomized studies evaluated clinical responses after palliative RT that was performed using conventional techniques and a permissible radiation dose for the spinal cord. Because conventional RT provides a relatively low biologically effective dose (BED), those dose schedules are often used for pain palliation rather than long-term tumor control. Up to 25% of the patients in these studies developed tumor progression within two years after treatment [5].
Stereotactic body radiotherapy (SBRT) is a highly precise and accurate method of radiotherapy that delivers high doses of radiation in a few or single fractions for the treatment of small-to-medium-sized extracranial tumors. SBRT has several theoretical advantages over conventional RT. In general, SBRT is delivered over fewer treatment days, which is more convenient for the patient than the typical 10 to 20 days needed for conventional fractionated RT. The short time required for SBRT reduces the delay in delivering systemic treatment, which usually is deferred during conventional RT to prevent the increased toxicity associated with concurrent therapy. The rapid dose fall off in SBRT can minimize the RT dose delivered to the spinal cord as well as to other adjacent critical organs, and therefore, toxicity related to SBRT should be less severe than the toxicity associated with conventional RT. Another advantage of SBRT over conventional RT is that the large RT doses typically delivered with SBRT allow escalation of the radiobiological dose to the tumor. Because of these advantages, SBRT has been increasingly used to treat patients with spinal metastases, particularly those with oligometastases, previous RT to the area of interest, or radioresistant tumors (soft tissue sarcoma, melanoma, renal cell carcinoma).
Since the use of SBRT for spinal lesions was first reported in 1995 [6], many institutions have published their initial experience with this approach. Because SBRT can deliver a BED that is higher than the BED of conventional RT, SBRT shows considerable promise, providing approximately an 85% rate of reduced pain, 90% rate of local control, and 80% rate of freedom from neurologic deficiency [78910].
Bone metastases from hepatocellular carcinoma (HCC) were previously relatively uncommon. However, because of the longer duration of tumor control at the primary site and improvements in imaging modalities, bone metastases from HCC are now identified much more frequently [11]. The incidence of bone metastases from HCC was reported to range from 3% to 40%, and 5% of patients with HCC were diagnosed with HCC after initially undergoing evaluation of symptoms due to bone metastasis [1213]. The most frequent site of bone metastasis from HCC is the vertebrae, where metastases are found at a rate of 68% to 72% [1112]. Although HCC is commonly thought to be a radiosensitive malignancy [14], the rate of response to SBRT for spinal metastases from HCC has not been reported. Bone metastases from HCC have some unique characteristics. About 13% to 54.8% of bone lesions have been found associated with a soft-tissue mass [111315]. An RT field determined by a bone scan or simple radiograph, which are used as traditional reference images for RT planning, may not cover the entire tumor, including the extended soft-tissue mass [16]. The resulting insufficient coverage might account for the observations that bone metastases from HCC are relatively resistant to RT. In this study, we report on our experience with SBRT for de-novo spinal metastases from HCC.
Materials and Methods
1. Patients
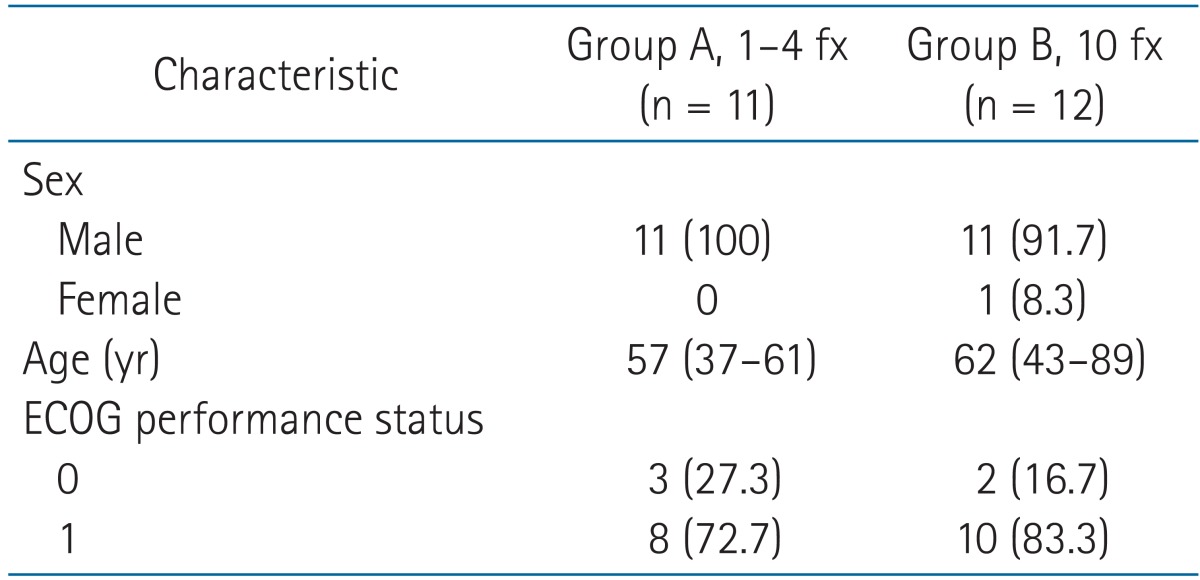
Between October 2008 and August 2012, 26 consecutive patients with 42 spinal lesions from primary HCC were treated with SBRT at Samsung Medical Center. Patient data were obtained from medical records and from imaging studies performed before SBRT and after SBRT at follow ups occurring at 1-to-2-month intervals. Inclusion criteria for this study were as follows: 1) histologically or clinically proven HCC; 2) confirmation of spinal metastases by computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography/computed tomography (PET/CT); 3) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 before the occurrence of neurologic symptoms; 4) age older than 20 years; and 5) medically inoperable or patient refused decompressive surgery. Patients who had undergone previous surgery or RT of the involved spinal region and those receiving concurrent systemic treatment were excluded. Three patients with six spinal lesions were excluded because there were no follow-up images that could be used to assess the radiologic response. Thus, a total of 23 patients with 36 lesions were enrolled in this retrospective study.
2. Treatment
Before RT, patients were generally seen by a neurosurgeon to discuss the option of decompressive surgery, if indicated. The enrolled study patients completed an evaluation for pain scoring and underwent a neurologic examination on their first visit before the start of SBRT. Each patient underwent MRI within two weeks before the start of treatment to assess the extent of the spinal lesion and cord compression. If a patient had not undergone MRI at the time of his or her initial visit and was suspected to have spinal metastases based on clinical features and CT imaging, MRI was performed on the day of CT simulation, with the patient in the same position and posture used for the simulation. The median time between MRI and CT simulation was 7 days. The MR image was fused with the CT simulation image after importation into Pinnacle3 treatment planning system (Philips Radiation Oncology Systems, Milpitas, CA, USA), in order to improve the delineation of the RT target and spinal cord. Each patient was immobilized using a vacuum immobilization system (Medical Intelligence, Schwabmunchen, Germany). Image acquisition was set at 2.5-mm slice thickness for both simulation CT and MRI.
The delineation of gross tumor volume (GTV) was aided by fusion of the simulation CT and MR images. Any MRI of the spinal lesion taken within two weeks prior to simulation can be utilized for image fusion. GTV was defined as any visible gross tumor on CT or MRI. The clinical target volume (CTV) was defined according to the extent of the spinal lesion suggested by the Radiation Therapy Oncology Group (RTOG) 0631. If the GTV involved a vertebral body only, the CTV included the involved vertebral body and both pedicles. If the GTV involved the pedicles, the CTV was more generous, or the CTV included the anterior and posterior elements of the spine. If the GTV involved the posterior element only, the CTV included the spinous process and lamina. If there was a paraspinal soft-tissue-tumor component, the CTV was expanded to at least 5 mm from the GTV.
The target dose was based on the discretion of the radiation oncologist, who took into consideration both the tumor volume and the organs at risk. A prescribed dose covered at least 80% to 90% of the defined GTV. There were 2 different dose fractionation RT schedules, which were chosen at the discretion of the treating physician and were based on the individual patient and characteristics of the spinal lesion. One schedule consisted of 1 to 4 treatment fractions and the other used 10 treatment fractions. The spinal cord or cauda equina was the primary dose-constraining organ. The tolerance doses were prescribed according to the RTOG protocol for 1, 3, and 4 RT fractions as follows: 10, 18, and 21 Gy, respectively, which were delivered to 10% of the volume of the partial spinal cord that included the target and cord extending 6 mm above and below the target. The absolute maximum dose to any part of the spinal cord was 14, 22, and 26 Gy, respectively, for a volume of 0.03 mL. For 10 RT fractions, 36 Gy, which corresponds to 50 Gy with the equivalent dose in 2-Gy fractions, was the maximum dose for the cord [17].
SBRT was delivered using the Novalis System (Brainlab AG, Heimstetten, Germany) for 1 to 4 fractions of RT or the TomoTherapy Hi-Art System (TomoTherapy Inc., Madison, WI, USA) for 10 fractions of RT. For setup verification, cone-beam CT and ExacTrac were used before each treatment by the Novalis system, and onboard megavoltage CT imaging was used for the TomoTherapy system.
3. Response evaluation
Patient response to treatment was evaluated 1 to 3 months after SBRT. The clinical response at follow up included the pain score, neurologic examination, and adverse events. Patients were asked to rate their pain intensity on a categorical scale of 0 to 10 (0 indicating absence of pain and 10 indicating worst pain possible). If a pain score at a follow-up visit was lower than the pain score of the patient before SBRT, the patient was considered to have obtained a decrease in pain.
Radiologic response was assessed using CT, MRI, or PET/CT performed at each follow-up visit. The radiologic response was evaluated according to Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.1 for CT, MRI, and PET Response Criteria in Solid Tumors (PERCIST) ver. 1.0 for PET/CT [1819]. Spinal-lesion progression-free survival was assessed using imaging data from the entire follow-up period.
Toxicity was assessed according to Common Terminology Criteria for Adverse Events (CTCAE), ver. 4.0. A compression fracture identified during a follow-up examination was defined as a new endplate fracture or new collapse deformity compared with the imaging findings before SBRT was performed.
4. Statistical analysis
Descriptive statistics were recorded as percentages for proportions and as medians and ranges for parametric values. Univariate analysis of RT schedules and effect on pain and radiologic response was performed using the chi-square test. Overall survival was calculated from the date of initiation of SBRT until death or final follow-up. Progression-free survival was defined as the date of initiation of SBRT to documented progression of a spinal lesion or death from any cause. The Kaplan-Meier method was used to estimate progression-free survival and overall survival, and the log-rank test was used to compare survival outcomes. Cox regression analyses was used to determine the impacts of the various following prognostic factors: gender, age, neurologic symptoms before RT, number of RT fractions, soft tissue extension, distance between cord and tumor, GTV, and percentage of average dose per prescribed dose.
The overall survival rates were stratified according to an index generated by recursive partitioning analysis (RPA), which was created by Chao et al. [20] to predict the survival rates of patients who had undergone SBRT for spinal metastases. Class 1 patients consisted of those whose time from the primary diagnosis to spinal metastasis (TPD) was >30 months and Karnofsky performance status (KPS) was >70. Class 2 patients had a TPD of >30 months and KPS of ≤70 or a TPD of ≤30 months and age <70 years. Class 3 patients had a TPD of ≤30 months and age ≥70 years. All statistical analyses were performed using SPSS software, version 20.0 (IBM, Armonk, NY, USA). A two-sided p-value of less than 0.05 was considered statistically significant.
Results
1. Characteristics of patients and spinal lesions
SBRT consisted of approximately 2 fractionation schedules, which were 1 to 4 fractions for group A lesions (n = 15) and 10 fractions for group B lesions (n = 21). The RT schedules were as follows: 1) 18 to 20 Gy in a single fraction for 13 lesions; 2) 36 Gy in 3 fractions for one lesion, 40 Gy in 4 fractions for one lesion; and 3) 50 Gy in 10 fractions for 21 lesions.
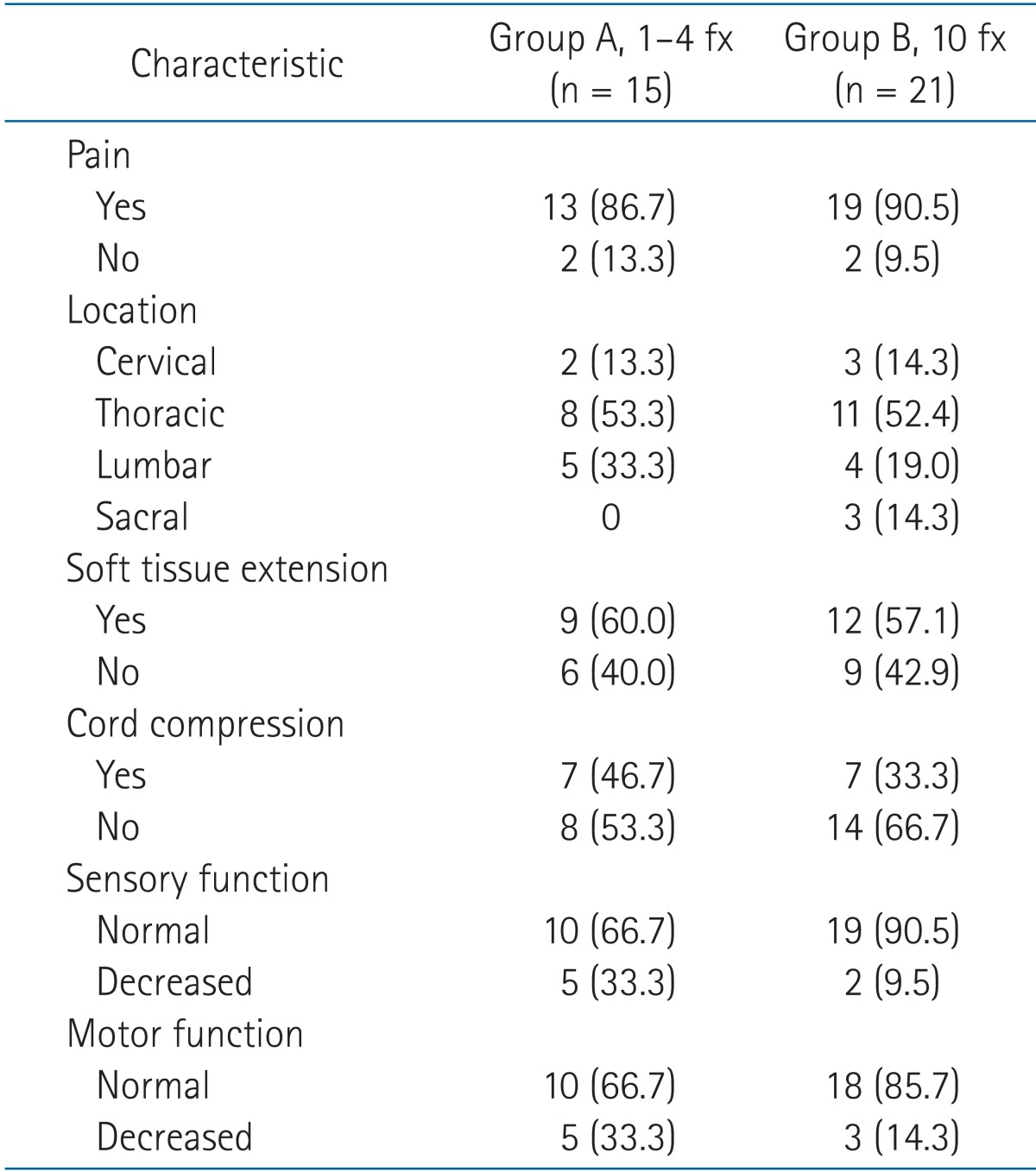
The median duration of follow up of the 23 patients was 7 months (range, 2 to 16 months). After exclusion of patients who died during the follow-up period, the median duration of follow up was 12 months (range, 9 to 16 months). The characteristics of patients and spinal lesions are shown in Tables 1 and 2, respectively. The differences between group A and group B patients and lesions were not significant. The ECOG performance status of five patients was 0 and of 18 patients was 1. The median time from the primary diagnosis to spinal metastasis was 16 months (range, 0 to 105 months). Thirty-two lesions were associated with pain before SBRT. Soft tissue extension accompanied 21 lesions. Sensory and motor functions were decreased in association with seven and eight lesions, respectively.
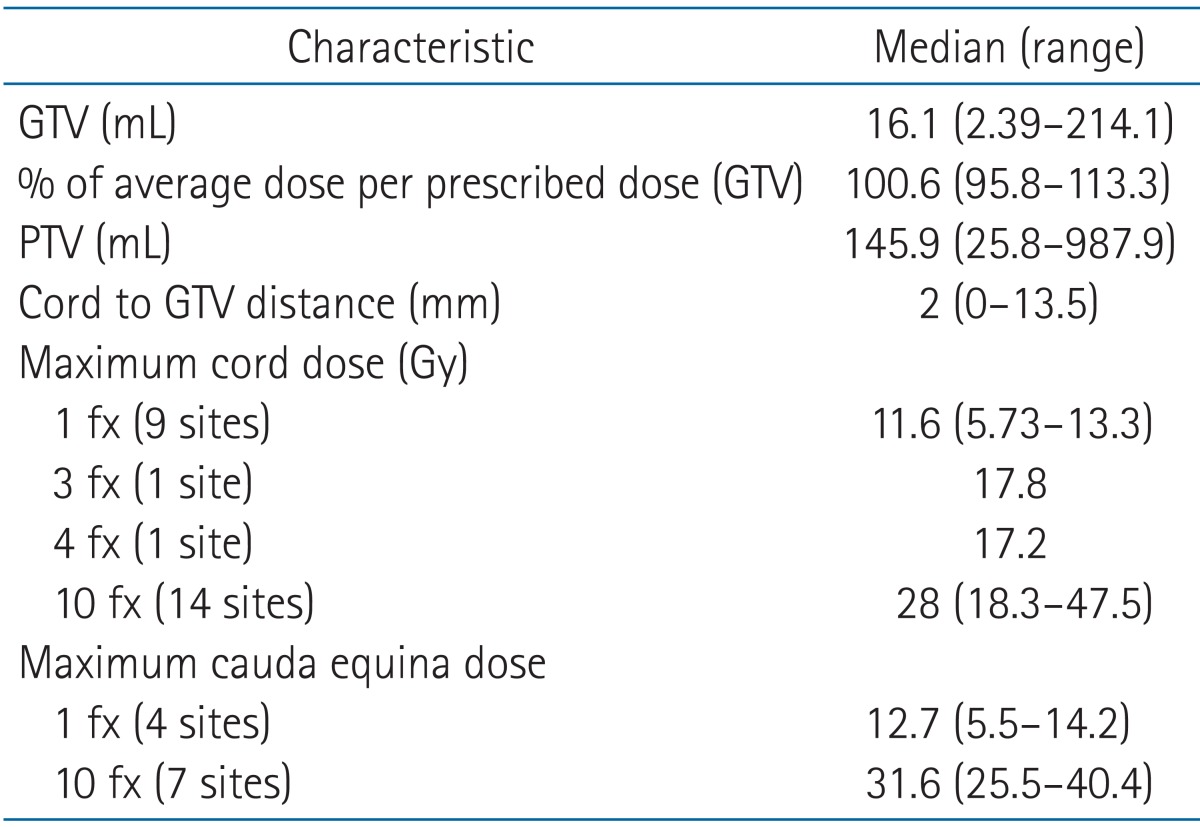
Table 3 shows the dosimetry values and associated characteristics. The median GTV was 16.1 mL (range, 2.39 to 214.1 mL). The median distance between the spinal cord and GTV was 2 mm (range, 0 to 13.5 mm). The tolerance doses to the spinal cord and cauda equina of every patient did not exceed the standard doses suggested by the RTOG.
2. Toxicity
Acute toxicity was evaluated during and within three months of SBRT. One patient developed nausea (grade 1). Esophagitis occurred in five patients (grade 1 in 3, grade 2 in 2). Grade 1 diarrhea occurred in one patient, and grade 2 leucopenia in one patient. There was no chronic toxicity related to SBRT. Compression fracture developed in association with nine lesions (25%) after treatment. The median time to the occurrence of a fracture was 2 months (range, 1 to 16 months). Treatment of the fractures consisted of vertebroplasty for 1 and conservative management for 8 fractures.
3. Response to SBRT
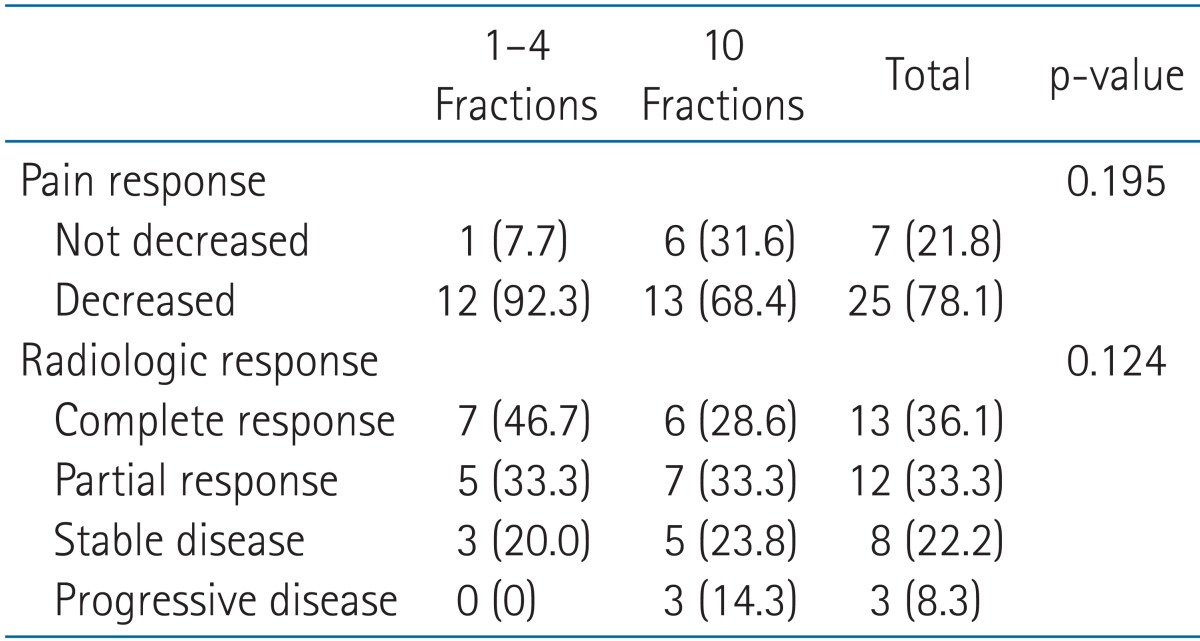
The effect of SBRT on pain associated with each lesion and the radiologic response of each lesion were evaluated within 3 months from SBRT according to RT schedule, and are shown in Table 4. There were no records on the effect of SBRT on the pain of four lesions, but the remaining 32 lesions were evaluated. The radiologic response rates were assessed using CT for 10, MRI for 7, and PET/CT for 9 patients.
All but 1 of the group A (92.3%) and 68.4% of the group B spinal lesions showed improvement in the degree of associated pain. In addition, the radiologic response rate was also better in group A. The radiologic response (complete and partial response) rates of the total, group A, and group B lesions were 69.4%, 80.0%, and 61.9%, respectively; and the complete response rates were 36.1%, 46.7%, and 28.6%, respectively. The differences in rates of pain relief and radiologic response between group A and B were not significant.
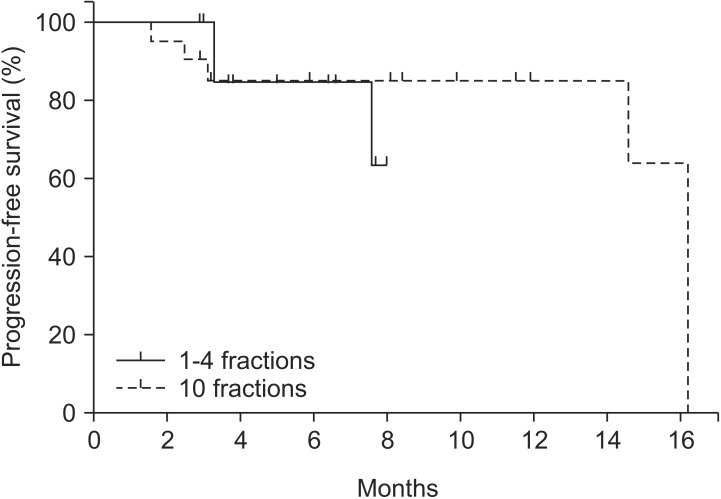
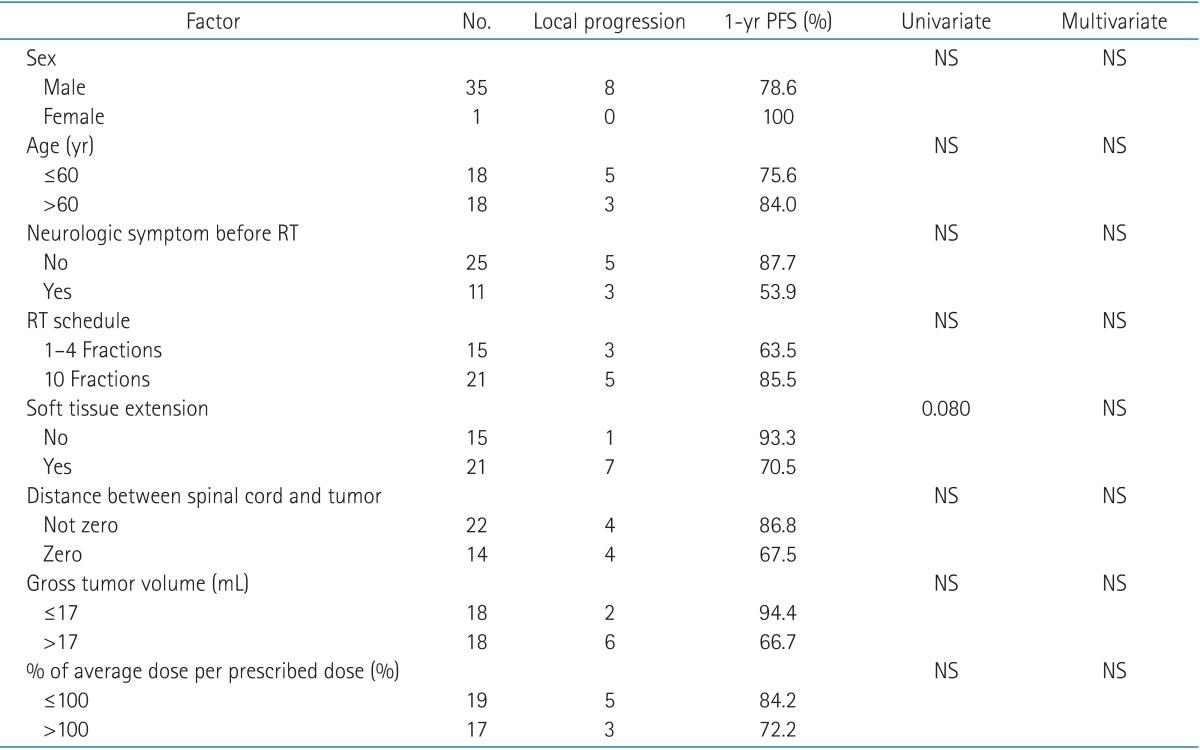
Of the eight lesions that showed local progression, seven lesions were local recurrence in the original SBRT field, and one lesion appeared in the margin of the SBRT field. The estimated 6-month and 1-year spinal-lesion progression-free survival rates were 84.6% and 78.6%, respectively. The difference between the group A and B lesions was not significant (Fig. 1). Univariate and multivariate analysis did not identify the following candidate prognostic factors to be significantly associated with survival: gender, age, neurologic symptoms before RT, distance between spinal cord and tumor, GTV, and percentage of mean dose per prescribed dose. Univariate analysis found marginal significance for soft-tissue extension (p = 0.080) (Table 5).
Among the 23 study patients, eight patients were neurologically intact at the initial visit for SBRT evaluation, and 15 patients presented with neurologic signs of decreased sensation (n = 7) and decreased muscle strength (n = 8). The neurological status of the eight patients who were intact before SBRT was unchanged after treatment. Among the seven patients who presented with sensory changes, three improved and four remained stable. Among the eight patients who presented with decreased motor strength, one improved and seven remained stable after SBRT.
4. Overall survival
During the follow-up period, 12 (52.2%) of 23 patients died of disease progression. The median survival time was 9 months (range, 2 to 16 months), and the 6-month and 1-year overall survival rates were 60.3% and 25.7%, respectively. The median overall survival was 9 months for the patients in RPA class 1 (n = 8), 7 months for those in RPA class 2 (n = 14), and 8 months for those in RPA class 3 (n = 1). The differences in survival rates among the 3 RPA classes of patients were not significant.
Discussion and Conclusion
The rates of improvement in pain after palliative RT for bone metastases from HCC have been reported to range from 70% to 99%. A dose-response relationship in association with pain relief has also been reported, and some patients who received high-dose RT for metastatic bone lesions from HCC also had longer survival [131521]. However, the response rate and dose-response relationship provided by SBRT for spinal metastases from HCC have not been reported.
Several institutions have reported excellent rates for pain relief and local control after SBRT for spinal metastases from a variety of primary cancers. In a prospective study performed at the Mayo Clinic, 85 lesions in 66 patients were treated by SBRT, with a median dose of 24 Gy in 3 fractions. The 1-year actuarial local control rates were 83.3% and 91.2% in patients with and without prior RT, respectively [22]. Gerszten et al. [9] prospectively evaluated 500 cases of spinal metastases. Out of this cohort, 86% of patients obtained long-term improvement in pain, and 88% of patients had long-term radiologic evidence of tumor control. Chang et al. [8] evaluated 74 spinal lesions in 63 patients who underwent SBRT for spinal metastases. The RT schedule was 30 Gy in 5 fractions for the first 32 patients, and 27 Gy in 3 fractions for the subsequent patients. During a median follow-up period of 21.3 months (range, 0.9 to 49.6 months), the actuarial 1-year spinal-tumor progression-free rate was 84%.
Our study, which evaluated patients with spinal metastases from HCC only, found that SBRT obtained results similar to the previous studies, including rates of improved pain and radiologic response, as well as the actuarial spinal-tumor progression-free survival. Interestingly, more patients treated by a shorter fraction schedule reported pain relief and obtained complete response within three months after treatment compared with patients treated by a longer fraction schedule, although the differences were not statistically significant. We think that a study with a larger number of patients will clarify the differences and show statistical significance. Several clinical experiences with single-dose SBRT for spinal metastasis showed that rapid pain relief and improvement of neurological function was obtained for patients with epidural compression [2324]. A shorter rather than longer SBRT fraction schedule is probably more suitable for patients who need rapid decompression because of a metastatic lesion compressing the spinal cord.
In our study, most local recurrences occurred within a CTV that received an adequate intended SBRT dose. These recurrences might have been due to the fact that the current dose of SBRT might be insufficient for controlling metastatic lesions from HCC. Investigators have reported that an increased dose (greater than 16 Gy) was required to achieve improvement in pain, especially for radioresistant tumors such as soft tissue sarcoma, melanoma, and renal cell carcinoma, as well as other tumors [232526].
The toxicity of high-dose SBRT for spinal metastasis has been reported to be acceptable. A retrospective study that matched 44 patients based on potential variables, and compared patients receiving external-beam RT with those receiving SBRT, did not find a statistically significant difference in pain relief [27]. However, the patients receiving external-beam RT had more acute toxicities (p = 0.01), including esophagitis in three patients, fatigue in one, and thrombocytopenia in one; whereas only one of the patients receiving SBRT developed nausea and vomiting. In our study, seven patients developed grade 1 or 2 gastrointestinal toxicity, and one developed grade 2 leucopenia. These toxicity results might be regarded as acceptable.
Spinal compression fracture is another complication that develops after SBRT. The incidence of compression fracture after SBRT has been reported to range from 11% to 39%, and the median time to fracture has ranged from 3 to 25 months [282930]. Because bone metastases from HCC tend to be associated with osteolytic soft-tissue masses [111315], and osteolytic bone lesions in the spinal body are a risk factor of compression fracture after SBRT [29]; it should not be surprising that a large proportion of spinal lesions from primary HCC will develop compression fractures after SBRT to the spine. Although 58% of the lesions in this study were treated by SBRT delivered in 10 fractions, compression fracture occurred in only 25% of the treated lesions. Special modalities, such as external fixation or vertebroplasty might be considered to prevent for fractures following SBRT for spinal metastases from primary HCC.
The prognostic factors of patients with spinal metastases include performance status, primary tumor, visceral metastases, motor function at presentation, and rapid tumor growth; however, most studies have primarily focused on identifying those patients who are ideally suited for surgical intervention [31323334]. The RPA classification uses TPD, KPS, and age to predict which patients undergoing spinal SBRT will benefit most from the treatment. The median overall survival times for patients stratified into RPA classes 1, 2, and 3 were 21.1 months, 8.7 months, and 2.4 months, respectively [20]. However, in this study, the differences in overall survival were not significant. A study that enrolls a larger number of patients would probably increase the probability of finding a significant difference in survival among the classifications.
Because the study was retrospective, the duration of follow-up was short, and the number of study patients was small. The overall survival rate was relatively poor, which might have resulted in some underestimation of actuarial spinal-lesion progression-free survival.
In conclusion, SBRT for spinal metastases from HCC was well tolerated, and was effective for pain relief and radiologic response in 78.1% and 69.4% of lesions, respectively. SBRT applied in fewer fractions appeared to provide higher rates of acute response than SBRT applied in more fractions. SBRT applied in fewer fractions also seemed to benefit patients with epidural compression. However, the differences seen in this study did not reach statistical significance, because of the small number of enrolled patients. Furthermore, because compression fractures develop at a high rate following SBRT for spinal metastases from primary HCC, careful follow up of the patient is required.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.