PIVKA-II as a surrogate marker for prognosis in patients with localized hepatocellular carcinoma receiving stereotactic body radiotherapy
Article information
Abstract
Purpose
This study aimed to determine the correlation between protein induced by vitamin K absence or antagonist-II (PIVKA-II) and stereotactic body radiotherapy (SBRT) in patients with hepatocellular carcinoma (HCC).
Materials and Methods
Sixty-one patients received SBRT between 2015 and 2020 with a median dose of 48 Gy (range, 39 to 60 Gy) with a median of 4 fractions. Changes in tumor markers before and after SBRT were analyzed.
Results
The median follow-up period was 31 months (range, 12 to 64 months). The estimated 2-year in-field failure-free survival, progression-free survival (PFS), and overall survival rates were 82.0%, 39.3%, and 96.7%, respectively. Patients with decreased PIVKA-II levels through SBRT had significantly few in-field failures (p = 0.005). Patients with PIVKA-II levels of ≤25 mAU/mL after SBRT had significantly long PFS (p = 0.004).
Conclusion
PIVKA-II could be a useful surrogate marker for response or survival outcomes in patients with localized HCC receiving SBRT.
Introduction
Hepatocellular carcinoma (HCC) is the 6th common cancer in both sexes, and has the 2nd most cancer mortality after lung cancer in Korea in 2018 [1]. Curative resection is the optimal treatment for patients with localized HCC. Several treatment options are available for patients who are ineligible for surgery, including transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), radiofrequency ablation (RFA), percutaneous ethanol injection therapy, and radiation therapy (RT). Selection of treatment modality depends on the tumor size, number, vascular invasion, and the patient’s performance status. In view of TACE, an objective response rate of 52.5% was reported in a systemic review [2]. However, common problems with TACE are refractoriness/failure and liver damage caused by repeated use [3].
External beam radiotherapy (EBRT) can be performed in various circumstances, from curative to palliative. Compared to conventional EBRT, stereotactic body radiotherapy (SBRT) delivers a highly conformal dose with a lower dose to the normal liver and is included in the National Comprehensive Cancer Network guidelines as a curative option [4]. SBRT for HCC has shown high rates of local control, ranging from 87% to 100% at 1 to 3 years in prospective clinical trials [5]. When evaluating the response to SBRT by an imaging study, we often have a difficult interpretation for differential diagnosis between response versus progression because of a focal liver reaction or continued regression even until 12 months after completion of SBRT. Therefore, we need another surrogate predictive marker for the evaluation of tumor response or survival after SBRT in addition to imaging studies. The role of biomarkers, such as alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) were investigated as prognostic factors in the treatment of HCC [6-10]. However, there are several advantages of PIVKA-II as a tumor marker over AFP as follows; first, it shows lower false-positive rate than AFP in the diagnosis of HCC because its level dose not increase non-specifically in most liver disease. Second, it could indicate earlier treatment response than AFP because of a relatively shorter half-life. Third, it is more related to the prognosis by reflecting biological characteristics of HCC such as tumor extent and vascular invasion. [11]. However, few studies have examined the role of biomarkers in predicting response or survival in cases of HCC receiving SBRT. Therefore, we conducted a study to determine the predictive role of AFP and PIVKA-II in SBRT outcomes.
Materials and Methods
1. Patient eligibility
We retrospectively reviewed the medical records of 61 patients with HCC who received SBRT between 2015 and 2020 at Chonnam National University Hwasun Hospital, Korea. Patients with the Eastern Cooperative Oncology Group performance score of ≤2, preserved liver function of Child-Pugh score (CPS) 5 to 7, and no prior history of radiotherapy were included. Exclusion criteria were as follows: presence of an extrahepatic metastatic lesion, a history of other malignancies, no regular imaging follow-up after SBRT, and other locoregional therapy within 6 months after SBRT without any sign of disease progression. All patients provided signed informed consent for treatment, and this study was approved by our Institutional Review Board of Chonnam National University Hwasun Hospital (No. CNUHH-2015-130).
2. SBRT planning and treatment
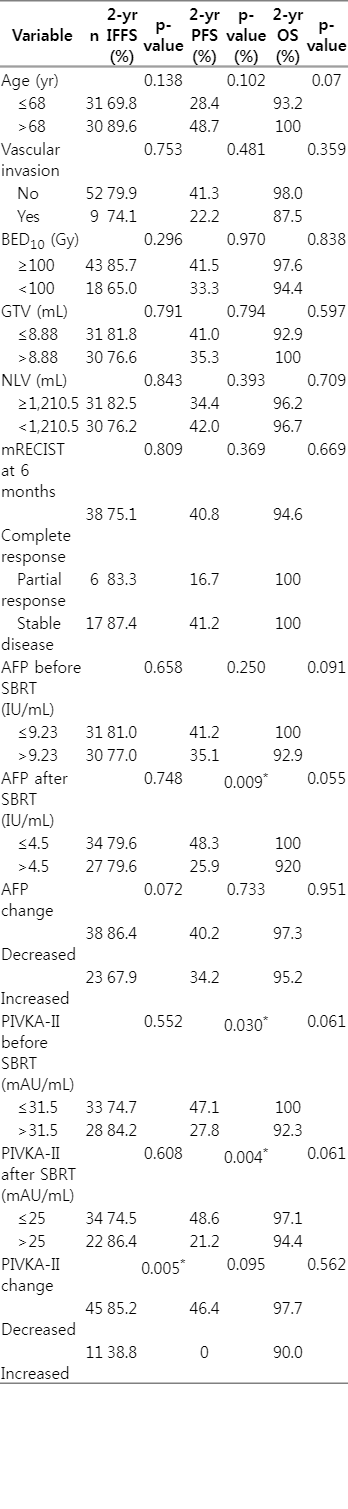
Before SBRT, all patients underwent a respiratory-correlated four-dimensional computed tomography (CT) simulation scan with immobilization using a vacuum cushion and Real-time Patient Management system (Varian Medical Systems, Palo Alto, CA, USA). The gross tumor volume (GTV) was defined as the lesion volume including lipiodol uptake, arterial enhancement, and necrotic portion using CT simulation images and pre-treatment triple-phase abdominal CT or magnetic resonance imaging (MRI). The internal target volume was regarded as equal to the GTV because of window images of 50%–60% gating phase. All organs-at-risk (OAR) were contoured on the simulation CT, and various dose constraints were prescribed in each OAR. The normal liver volume (whole liver volume minus GTV) receiving less than 15 Gy (rV15 Gy) in 3 fractions or 17 Gy (rV17 Gy) in 4 fractions should be spared over at least 700 mL as possible. Maximum doses to the stomach, duodenum, small bowel, colon, and spinal cord, etc., were limited depending on fraction numbers. The planning target volume (PTV) was defined as GTV plus individualized margins (0.5 to 1 cm) to account for the movement of enhanced mass within the gated phases of respiration and a set-up error. The total dose was prescribed to cover at least 95% of the PTV (Fig. 1). SBRT was delivered in 3 or 4 fractions using 6 MV photons every other day to reduce nearby intestinal toxicity. Dose fractionation was prescribed as equal to or greater than 80 Gy of biologically effective dose with an α/β ratio of 10 (BED10). All patients underwent cone-beam CT to verify skeleton alignment and On-Board Imager (Varian Medical Systems) for diaphragm matching in the anterior-posterior direction at the end-exhale phase on each treatment day.
The treatment planning of stereotactic body radiotherapy for patient with hepatocellular carcinoma. The prescription dose was 51 Gy in 3 fractions in this case. The lowest isodose curve indicates 15 Gy and the normal liver volume (whole liver volume minus gross tumor volume) receiving less than 15 Gy (rV15 Gy) was 1,178 mL.
3. Patient follow-up and response assessment
After treatment, follow-up examinations including physical examinations, complete blood counts, biochemical profiles, tumor markers, and imaging studies were assessed at 1–3-month intervals. The changes in the two tumor markers, AFP and PIVKA-II, were calculated as the difference between the level before SBRT and the lowest level within 6 months after SBRT. At 6 and 12 months after SBRT, images were assessed according to modified Response Evaluation Criteria in Solid Tumors (mRECIST) [12]. In-field failure (IFF) was defined as recurrence or increased volume of the treated lesion. In-field failure-free survival (IFFS) was estimated from the date the start of SBRT to the date of IFF. Overall survival (OS) was estimated from the date of the start of SBRT to the date of death or the last follow-up. Progression-free survival (PFS) was estimated from the date of the start of SBRT to the date of failure of any kind, death of any cause, or the last follow-up.
Both univariate and multivariate analysis were performed with the same variables for determining any prognostic factor. Kaplan-Meier models were used for the survival analysis of all potential factors that affected the treatment results and were tested using the log-rank test. A Cox proportional hazards model was used for the multivariate analysis. For the statistical analysis, p-values less than 0.05 were considered significant. All statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY, USA).
Results
1. Patient characteristics and treatment
The characteristics of the patients, tumor, and treatment-related factors are summarized in Table 1. Most patients were CPS class A, and only two patients were CPS class B7. Vascular involvement was observed in nine patients (14.8%). Fifty-seven patients (93.4%) underwent local therapies before SBRT. Five patients (8.1%) underwent curative resection before SBRT. Fifty-six patients (91.8%) underwent TACE (median 2; range, 1 to 13) prior to SBRT. Twenty-four patients (39.3%) underwent RFA (median 1; range, 1 to 7) prior to SBRT. Only one patient received HAIC. According to the target or non-target lesions of SBRT, detailed breakdown of history of previous treatment was shown in Table 2. The median total doses of SBRT and BED10 were 48 Gy (range, 39 to 60 Gy) and 112.5 Gy (range, 80 to 180 Gy), respectively. The median levels of serum AFP and PIVKA-II before SBRT were 9.23 (range, 1.35 to 2,446) and 31.5 (range, 11 to 15,352), respectively. All 61 patients had AFP tested before SBRT, and 56 patients were tested for PIVKA-II. After SBRT, AFP levels in 38 out of 61 patients and PIVKA-II levels in 45 out of 56 patients decreased. The median decrease in AFP level was -56% (interquartile range, -87.1% to -21.4%) and that of PIVKA-II was -34.5% (interquartile range, -80.7% to -11.4%). The median lowest level was 4.31 (range, 1.19 to 3,055) and 21.5 (range, 10 to 49,812) for AFP and PIVKA-II, respectively within 6 months after SBRT.
2. Treatment response and survival
The median follow-up time was 31 months (range, 12 to 64 months). According to the mRECIST by imaging studies at 6 months, there were 38 patients (62.3%) with complete response (CR), 6 (9.8%) achieved partial response (PR), and 17 (27.9%) achieved stable disease (SD). At 12 months, there were 45 patients (73.8%) with CR, 3 (4.9%) achieved PR, 11 (18%) achieved SD, and 2 (3.3%) had progressive disease. Patients with a lower level of PIVKA-II after SBRT had a tendency to achieve a higher CR at 6 months. Of the 56 patients, 34 patients had PIVKA-II level of ≤25 mAU/mL after SBRT, of which 25 patients (73.5%) achieved CR, while only 11 of 22 patients (50%) with a PIVKA-II level >25 mAU/mL achieved CR (p = 0.053). A low level of AFP after SBRT or changes in PIVKA-II or AFP levels by SBRT had no statistically significant association with treatment responses.
At the time of the analysis, the median time to IFF was 12 months (range, 3 to 24 months), while the median PFS was 8 months. The estimated 2-year IFFS, PFS, and OS rates in all patients were 82.0%, 39.3%, and 96.7%, respectively (Fig. 2). The survival differences between response subgroups by mRECIST at 6 or 12 months did not show any statistical significance because we could suppose that additional treatments were intervened according to the response after SBRT or delayed response after 6 months. Of the 10 patients who achieved PR or SD at 6 months after SBRT, six achieved delayed CR at 12 months without any additional treatment.
3. Prognostic factors for survivors
In the univariate analysis, decreased changes in PIVKA-II levels after SBRT were significantly associated with better IFFS (p = 0.005). The PIVKA-II level of ≤31.5 mAU/mL before SBRT was significantly associated with better PFS (p = 0.03). An AFP level of ≤4.5 IU/mL after SBRT and a PIVKA-II level of ≤25 mAU/mL after SBRT were found to be significantly associated with a high PFS (Table 3, Fig. 3). In the multivariate analysis, a decreased change in PIVKA-II level after SBRT was significantly associated with IFFS (p = 0.011), and a PIVKA-II level of ≤25 mAU/mL after SBRT was significantly associated with a good PFS (p = 0.006) (Table 4). Two-year PFS in the post-SBRT PIVKA-II level of ≤25 mAU/mL group was 48.6% and 21.2% in the >25 mAU/mL group (p = 0.004).

In-field failure-free survival according to AFP change (A) and PIVKA-II change (B), and progression-free survival according to serum AFP after SBRT (C) and serum PIVKA-II after SBRT (D). AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; SBRT, stereotactic body radiotherapy.
4. Failure patterns and toxicities
Thirty-nine patients (63.9%) showed progression. Most of the first progression occurred at the intrahepatic out-field site (28/39, 71.8%) (Fig. 4) and the level of PIVKA-II increased again in 26 out of 39 patients (66.7%). Re-elevation of PIVKA-II level in these patients seemed to be more related to out-field failure rather than IFF. Only four patients failed at the SBRT site as the first progression, and two of these had out-field failure simultaneously. Nine patients had disease progression at the extrahepatic site without liver disease progression (Fig. 4), and the first sites of extrahepatic failure were the lung (n = 4), lymph nodes (n = 3), and bone (n = 2). Three patients died, and all deaths were caused by disease progression. Eleven patients (18%) had IFF at the SBRT site, and their characteristics are summarized in Table 5. Compared to the in-field control group, all clinical, tumor, and treatment factors were not statistically significantly different on Student t-test. However, the median percent changes in AFP levels after SBRT in each group showed a different trend: 36% and -22% in the IFF and in-field control groups, respectively (p = 0.089). The median percent changes in PIVKA-II levels after SBRT in each group also showed a different trend: -5% and -26% in the IFF and in-field control groups, respectively (p = 0.330).
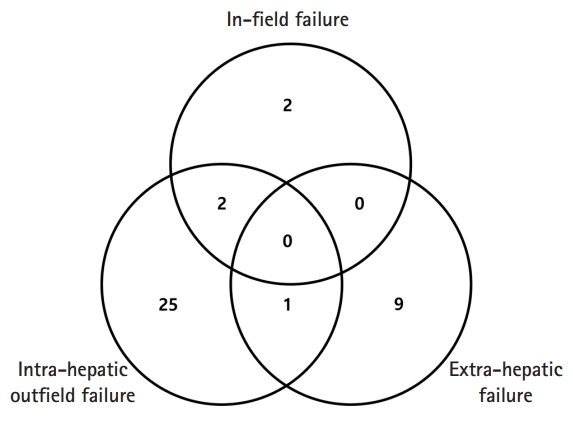
First failure patterns of all failure patients. Intra-hepatic out-field failure was defined as newly appeared or aggravation of lesion within liver outside the treated lesion, and extra-hepatic failure was defined as recurrent disease at any site outside the liver.
None of the patients developed radiation-induced liver disease (RILD) or non-classic RILD during the follow-up. Two patients experienced worsening of CPS class A to B. One patient had gastrointestinal toxicity of melena and underwent argon plasma coagulation at the gastric antrum. He received palliative RT of 40 Gy in 16 fractions for recurrent portocaval and para-aortic nodes, the field of which overlapped partially with the previous SBRT field.
Discussion and Conclusion
This study showed results comparable to those of historical studies in terms of local control [5]. SBRT has been an effective local therapy for patients with Barcelona Clinic Liver Cancer staging A and even for advanced C or with CPS class B or C in selected cases [13,14]. Several studies have reported treatment outcomes and prognostic factors for SBRT. Predictive factors, such as the tumor size, multiplicity, and SBRT dose are associated with disease progression [15-17]. For OS, CPS class, portal vein thrombosis, and extrahepatic spread are prognostic factors [18].
AFP and PIVKA-II serve as tumor markers in the diagnosis of HCC and as prognostic factors [19]. Clinical implication is different between both markers. The AFP indicates extent of local tumor burden while PIVKA-II shows close association with tumor invasiveness such as vascular invasion or metastases [11]. High AFP levels are associated with lower local control after SBRT [16]. Uemoto et al. [20] assessed appropriate CTV for SBRT and suggested that high AFP levels may indicate microscopic invasion around the tumor, resulting in local failure. AFP levels before SBRT and changes in AFP levels after SBRT were investigated. AFP normalization within 3 months after SBRT is a favorable prognostic factor for OS and PFS [6]. In this study, AFP normalization was defined as an AFP level of 20 ng/ml or less. On the contrary, there is a report that transient increases in AFP or PIVKA-II levels at 12 months follow-up after proton therapy (PT) were not statistically significantly associated with treatment response because tumor lysis, acute hepatitis, or cell regeneration could induce elevation [8]. In a meta-analysis of 29 studies that were stratified by AFP response after treatment, AFP response after various treatments could be a useful prognostic factor for HCC patients [21].
In a Japanese SBRT study, serum the PIVKA-II level (>35 vs. <35 mAU/mL) was a significant predictive factor for OS but not for PFS. In the PIVKA-II level <35 mAU/mL group, median survival was not reached in the follow-up of 2,250 days [7]. In a retrospective study of the outcomes of recurrent HCC patients undergoing TACE, PIVKA-II was measured immediately before TACE and approximately 1 month later. A significant correlation between PIVKA-II and treatment response was found, and an elevated PIVKA-II level was an independent prognostic factor for poor survival [22]. In a recent study, the prognostic role of AFP and PIVKA-II in HCC patients receiving anti-PD-1 immunotherapy was investigated. Serum levels were measured 1 week before and 5–7 weeks after immunotherapy. Serum response was defined as a >50% reduction in serum concentration. PIVKA-II responders had a longer PFS of 10.9 months compared to 4.5 months in PIVKA-II non-responders [9]. No study has verified the usefulness of PIVKA-II for predicting prognosis after SBRT. In our study, increased PIVKA-II levels after SBRT were significantly associated with IFF, and PIVKA-II levels of ≤25 mAU/mL after SBRT were significantly associated with PFS.
There were several limitations to this retrospective study. First, all patients were not tested for PIVKA-II before SBRT, and there might be a bias in the selection of patients. However, most patients underwent consecutive marker studies before and within 6 months after SBRT. Second, we calculated the change in biomarker levels by using the lowest value within 6 months without setting the specific time point. Each time point of achieving the lowest level would imply different treatment responses and tumor cell kinetics. Further studies are needed to evaluate the role of markers at consistent time points after SBRT.
In conclusion, patients with a decreased change or a lower level of PIVKA-II after SBRT showed good tumor control and survival. PIVKA-II could be a useful surrogate marker for response or survival outcomes in patients with localized HCC receiving SBRT.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by a grant from Chonnam National University Hwasun Hospital Institute for Biomedical Science (No. HCRI 15002-1).