 |
 |
AbstractPurposeFor recurrent esophageal cancer after primary definitive radiotherapy, no general treatment guidelines are available. We evaluated the toxicities and clinical outcomes of re-irradiation (re-RT) for recurrent esophageal cancer.
Materials and MethodsWe analyzed 10 patients with recurrent esophageal cancer treated with re-RT after primary definitive radiotherapy. The median time interval between primary radiotherapy and re-RT was 15.6 months (range, 4.8 to 36.4 months). The total dose of primary radiotherapy was a median of 50.4 Gy (range, 50.4 to 63.0 Gy). The total dose of re-RT was a median of 46.5 Gy (range, 44.0 to 50.4 Gy).
ResultsThe median follow-up period was 4.9 months (range, 2.6 to 11.4 months). The tumor response at 3 months after the end of re-RT was complete response (n = 2), partial response (n = 1), stable disease (n = 2), and progressive disease (n = 5). Grade 5 tracheoesophageal fistula developed in three patients. The time interval between primary radiotherapy and re-RT was less than 12 months in two of these three patients. Late toxicities included grade 1 dysphagia (n = 1).
IntroductionEsophageal cancer is usually an advanced disease at presentation, and is associated with poor prognosis regardless of the introduction of effective multimodal treatment regimens. The common failure of definitive chemo-radiotherapy is locoregional. The local recurrence rate at the first site was reported to be approximately 44-61% [1,2]. All patients with locoregional recurrence died within 1 year without treatment [3]. If recurrence occurs, the 5-year survival rate drops to 0-11% [4-6].
Few curative or palliative treatment options exist for recurrent or persistent esophageal cancer after primary definitive radiotherapy. In patients with locoregional recurrence after primary definitive radiotherapy, esophagectomy can be considered if resectable and medically operable. The rate of salvage esophagectomy in patients treated with primary definitive radiotherapy with curative intent was reported to be 4-29%. However, a high hospital mortality rate was reported (about 8-15%). Long-term survival after salvage esophagectomy was related to resection without residual tumors (R0). The 5-year survival rate after salvage esophagectomy was reported to be up to 25-35% [3,7]. For unresectable or medically inoperable esophageal cancer, best supportive care is recommended (including external beam radiation therapy, intraluminal brachytherapy, chemotherapy, hyperthermia, laser, dilatation, endoscopic mucosal resection, and stent insertion) [8,9].
In patients with locoregional recurrence after primary definitive radiotherapy, re-irradiation (re-RT) is used with caution because of the increased probability of normal tissue complications [10]. Few reports exist regarding re-RT of recurrent or persistent esophageal cancer after primary definitive radiotherapy using external beam or intraluminal brachytherapy [10-14]. The results of re-RT of the esophagus were somewhat favorable, despite severe toxicities such as tracheoesophageal fistula, esophageal perforation, esophageal stricture, fatal arterial hemorrhage, and pericardial effusion. However, no general treatment guidelines concerning re-RT of the esophagus are available.
The purpose of this study was to evaluate the toxicities and clinical outcomes of re-RT for recurrent esophageal cancer after primary definitive radiotherapy.
Materials and Methods1. Patient selectionFrom January 2007 to November 2011, a total of 267 patients with esophageal cancer were treated with external beam radiotherapy at our institution. Seventeen of the patients were re-irradiated at the esophagus. Re-RT of the esophagus was determined at a multidisciplinary team conference at the Esophageal Cancer Clinic. Of the 17 patients, 3 refused salvage surgery at the time of recurrence; 14 were inoperable considering the medical condition and disease extent at the time of recurrence, so re-RT was chosen as a second treatment option.
One patient with perigastric lymph node recurrence was excluded. Another patient with no follow-up information after completion of re-RT was excluded. Five patients with boost irradiation were excluded. The total dose of boost irradiation was less than 30 Gy in these five patients. Ten patients with re-RT to the recurrent esophageal cancer after primary definitive radiotherapy were analyzed retrospectively.
The diagnosis of recurrence (or re-growth) was based on tissue biopsy, esophagography, endoscopic gastroduodenoscopy (EGD), endoscopic ultrasound (EUS), 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT), and/or chest CT. A recurrent (or re-growing) lesion was defined as a progressive lesion after a partial response (PR) or stable disease (SD) treatment response with primary radiotherapy or a newly developed lesion after a complete response (CR) with primary radiotherapy.
The Eastern Cooperative Oncology Group (ECOG) performance status and tumor/node/metastasis (TNM) stages (American Joint Committee on Cancer [AJCC], 7th edition) were evaluated at the time of primary radiotherapy, and re-RT.
2. RadiotherapyThe total doses of primary radiotherapy and re-RT are listed in Tables 1 and 2. Ten patients were re-irradiated with an external beam with 6- or 10-MV energy using CT-based three-dimensional (3D) treatment planning. The re-RT modality included a 3D conformal radiotherapy using linear accelerator (n = 7) and intensity-modulated radiation therapy using helical tomotherapy (n = 3).
The target delineations of recurrent esophageal cancer were as follows: the recurrent gross tumor lesion was delineated as the gross tumor volume (GTV); the clinical target volume (CTV) was defined as GTV plus a 1.5- to 2.5-cm margin; and CTV plus a 5- to 10-mm margin was defined as the planning target volume (PTV).
The number of beams at re-RT in the linear accelerator was 3-6 (considering the normal organ toxicity). The total cumulative dose to the spinal cord was limited not to exceed the maximal dose of 45 Gy. However, in some cases, a total cumulative dose to the spinal cord greater than 45 Gy was accepted, considering the time interval between primary radiotherapy and re-RT, and the volume of irradiation. The length of the re-irradiated esophagus, which was defined as the re-irradiated esophagus receiving greater than or equal to 90% of the prescribed dose, was calculated [11].
3. Evaluation of objective response, symptom relief, and toxicityThe objective treatment responses were evaluated using Response Evaluation Criteria in Solid Tumors (RECIST). The follow-up evaluations were performed using endoscopy, endoscopy-based biopsy, PET-CT, or CT every 1-3 months. CR was defined as the disappearance of all target lesions. PR was defined as at least a 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as a reference the baseline sum of the LD. SD was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD), taking as a reference the smallest sum of the LD since the treatment started. PD was defined as at least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum of the LD recorded since the treatment started or the appearance of one or more new lesions.
The degree of treatment-related toxicity was evaluated by the treating physician and recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 3.0. Toxicity was defined as acute (during therapy and within 3 months after therapy) and late (over 3 months after the completion of therapy). Local failure was defined as PD of the re-irradiated esophagus or regional lymph node.
Results1. Patient characteristics and treatmentPatient characteristics at diagnosis are shown in Table 1. Nine patients were male. The median age was 73.5 years (range, 55 to 85 years). The primary tumor histology was squamous cell carcinoma in all patients (n = 10). Eight patients had moderately differentiated disease, and two had poorly differentiated disease. Clinical stages at primary irradiation were as follows: stage I (n = 1), stage II (n = 5), and stage III (n = 4). Nine of 10 patients were treated with primary irradiation plus concurrent chemotherapy. One patient was treated with primary irradiation and sequential chemotherapy.
The median interval between the primary radiotherapy and re-RT was 15.6 months (range, 4.8 to 36.4 months) (Table 2). Re-RT fields were overlapped partially or were within the initial irradiation fields. The median longitudinal length of re-RT was 12.5 cm (range, 9.0 to 16.0 cm). The biologically equivalent dose (BED) can be obtained using the following formula: tumor cell survival following n fractions, each of dose d,
BED = nd[1 + d / (Оұ / ОІ)]
The cumulative dose of radiation was the sum of the total dose of primary radiotherapy and re-RT. When the total doses of radiation was converted into BED using an Оұ/ОІ ratio of 3 based on a linear-quadratic model, BED3 ranged from 152.6 to 181.4 Gy (median, 161.3 Gy).
Three of 10 patients were treated with concurrent chemotherapy during the course of re-RT. Chemotherapy regimens with re-RT were as follows: docetaxel plus ifosfamide (n = 1), 5-fluorouracil plus leucovorin (n = 1), and taxol (n = 1) (Table 2).
2. Treatment responseThe median follow-up period was 4.9 months (range, 2.6 to 11.4 months). All 10 patients completed the planned re-RT dose. The tumor responses at 3 months after the end of re-RT were CR (n = 2), PR (n = 1), SD (n = 2), and PD (n = 5) (Table 2).
Patient #2 was treated with neoadjuvant concurrent chemoradiotherapy (50.4 Gy with a daily dose of 1.8 Gy, initially). At 3 months after primary radiotherapy, the treatment response was PR, although an endoscopic biopsy report showed no malignant cell. However, patient #2 refused the definitive surgery and treated with six cycles of sequential chemotherapy with TS-1/cisplatin. Nine months after primary radiotherapy, increased uptake was observed in the primary middle and lower thoracic esophagus regions on PET-CT and a progressive growing lesion on EUS; however, the biopsy report was free of tumors. This lesion was re-irradiated with a total dose of 45 Gy with a daily dose of 1.8 Gy. Tracheoesophageal fistula and esophageal perforation developed 1 day before completion of re-RT, and pericardial effusion developed 1 month after completion of re-RT. Patient #2 died at 3.2 months after initiation of re-RT.
Patient #4 was treated with concurrent chemo-radiotherapy (50.4 Gy with a daily dose of 1.8 Gy, initially). At 3 months after primary radiotherapy, the treatment response was PR, although endoscopic biopsy report was no malignant cell. At 5 months after primary radiotherapy, there was ulcerofungating mass lesion on the upper thoracic esophagus, and the biopsy report was squamous cell carcinoma with moderate differentiation. This lesion was re-irradiated with a total dose of 50.4 Gy with a daily dose of 1.8 Gy. Tracheoesophageal fistula and esophageal perforation developed 20 days after completion of re-RT, and patient #4 died at 2.7 months after initiation of re-RT.
Patient #9 was treated with concurrent chemo-radiotherapy of 50.4 Gy with a daily dose of 1.8 Gy, initially. At 3 months after primary radiotherapy, the treatment response was PR. At 15 months after primary radiotherapy, a progressive lesion was found on the primary site. This lesion was re-irradiated with concurrent chemotherapy, a total dose of 50.0 Gy with a daily dose of 2.0 Gy. Tracheoesophageal fistula developed 3 days after completion of re-RT. Aspiration pneumonia and lung abscess developed sequentially thereafter. Patient #9 died at 2.6 months after initiation of re-RT.
3. ToxicityAcute toxicities included grade 3 hematologic disorder (n = 1) and grade 5 esophageal perforation, tracheoesophageal fistula (n = 3) (Table 3 and Fig. 1). Three patients (patients #2, #4, and #9) died due to esophageal perforation and tracheoesophageal fistula within 2 months after completion of re-RT. The acute dysphagia grades for pre- and post-re-irradiation were grade 0-grade 0 in two patients, grade 0-grade 1 in one patient, grade 1-grade 1 in one patient, and grade 2-grade 2 in three patients. In patients #2, #4, and #9, the dysphagia grade after re-RT could not be evaluated because of acute severe complications (esophageal perforation and tracheoesophageal fistula). Late toxicities included grade 1 dysphagia (in patient #3).
Discussion and ConclusionFew reports exist concerning re-RT of recurrent esophageal cancer after primary radiotherapy plus esophagectomy [4]. In addition, few reports exist regarding re-RT of recurrent or persistent esophageal cancer after primary definitive radiotherapy (Table 4). In the present study, we evaluated the toxicities and clinical outcomes after re-RT of recurrent esophageal cancer after primary definitive radiotherapy.
Yamaguchi et al. [11] reported 31 patients with recurrent or persistent esophageal cancer treated with 3D conformal re-RT (Table 4). Of the 31 patients, 14 underwent regional hyperthermia during the re-RT. The curative group (n = 11) was defined as patients without symptoms or who had mild dysphagia, without distant metastasis, and good performance. All other patients were classified into the palliative group. In the palliative group, severe esophageal toxicities were more common in the patients with advanced T stage (T3 or T4) at the time of re-RT.
Nonoshita et al. [12] reported 6 patients with recurrent esophageal cancer after external radiotherapy (median dose, 60 Gy). T stage at the time of primary radiotherapy and recurrence was T1 in all patients. Re-RT with high-dose-rate brachytherapy (HDRB) was performed once a week with a dose of 4 or 5 Gy per fraction (median total dose, 20 Gy). No toxicity was observed over grade 3. The median overall survival was 30 months.
Teli et al. [13] reported a prospective and randomized study that included 34 patients who were palliated with re-RT and 35 patients who refused re-RT and received peroral demand dilatation alone. Patients in the re-RT group showed better and more sustained improvement in their grade of dysphagia. The median overall survival period in the re-RT group was 7.9 months compared with 3.1 months in the non-re-irradiated group. Six tracheoesophageal perforation cases were reported in the non-re-irradiated group, whereas no tracheoesophageal perforation was reported in the re-irradiated group.
In the Harms et al. [10] study, 16 patients with inoperable recurrence from esophageal cancer were re-irradiated using pulsed dose rate (PDR) brachytherapy. Patients treated with a weekly 5 Gy daytime schedule (0.5 Gy/pulse/hr, total dose 15-20 Gy). After a median follow-up of 8 months, 3 patients showed a complete remission, and 5 showed a partial remission. Three patients with uncontrolled locoregional disease showed grade 4 complications (tracheoesophageal fistula, n = 2 or fatal arterial bleeding, n = 1).
In the literatures, the median overall survival after re-RT of recurrent esophageal cancer after primary definitive radiotherapy was 6.5-30.0 months [11,12] (Table 4).
In the present study, toxicities greater than grade 3 (non-hematologic) were reported in 3 patients; fatal tracheoesophageal fistula and esophageal perforation. Other studies also reported tracheoesophageal fistula and esophageal perforation (Table 4). Yamaguchi et al. [11] reported that advanced T stage (T3 or T4) at the time of recurrence was significantly related to development of severe toxicities greater than grade 3 (p = 0.03). In the study of Nonoshita et al. [12], T stage at the time of recurrence was T1 in all patients (n = 6), and toxicities greater than grade 3 were not reported.
One re-RT study reported that all newly developed tracheoesophageal fistulas (n = 3) after re-RT were the result of disease [15]. Harms et al. [10] reported that three patients (19%) presented with grade 4 complications (tracheoesophageal fistula, n = 2 or fatal arterial bleeding, n = 1), and all these patients suffered from uncontrolled locoregional disease. Thus, it remains unclear whether severe toxicity greater than grade 3 is truly treatment-related or due to progressive disease [10].
In the re-RT of recurrent esophageal cancer after primary radiotherapy, the total dose of re-RT, time interval between primary radiotherapy and re-RT, extent of disease progression, treatment modality, concurrent chemotherapy, recurred tumor T stage, and irradiated volume of esophagus can affect the toxicity.
In conclusion, because of the small number of patients, it is difficult to generalize prognostic factors related to severe toxicity with re-RT. Re-RT of recurrent esophageal cancer after primary radiotherapy can cause severe toxicity.
AcknowledgmentsThis study was supported by a grant from the Korea Healthcare Technology R&D Project, the Ministry for Health, Welfare & Family Affairs, Korea (A084120).
References1. Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593вҖ“1598, PMID: 1584260.
2. Araujo CM, Souhami L, Gil RA, et al. A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer 1991;67:2258вҖ“2261, PMID: 1707338.
3. Tachimori Y. Role of salvage esophagectomy after definitive chemoradiotherapy. Gen Thorac Cardiovasc Surg 2009;57:71вҖ“78, PMID: 19214447.
4. Fakhrian K, Gamisch N, Schuster T, Thamm R, Molls M, Geinitz H. Salvage radiotherapy in patients with recurrent esophageal carcinoma. Strahlenther Onkol 2012;188:136вҖ“142, PMID: 22218502.
5. Yano M, Takachi K, Doki Y, et al. Prognosis of patients who develop cervical lymph node recurrence following curative resection for thoracic esophageal cancer. Dis Esophagus 2006;19:73вҖ“77, PMID: 16643173.
6. Shioyama Y, Nakamura K, Ohga S, et al. Radiation therapy for recurrent esophageal cancer after surgery: clinical results and prognostic factors. Jpn J Clin Oncol 2007;37:918вҖ“923, PMID: 18211982.
7. Morita M, Kumashiro R, Hisamatsu Y, et al. Clinical significance of salvage esophagectomy for remnant or recurrent cancer following definitive chemoradiotherapy. J Gastroenterol 2011;46:1284вҖ“1291, PMID: 21818602.
8. Yano T, Muto M, Hattori S, et al. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy 2008;40:717вҖ“721, PMID: 18773340.
9. Hattori S, Muto M, Ohtsu A, et al. EMR as salvage treatment for patients with locoregional failure of definitive chemoradiotherapy for esophageal cancer. Gastrointest Endosc 2003;58:65вҖ“70, PMID: 12838223.
10. Harms W, Krempien R, Grehn C, Berns C, Hensley FW, Debus J. Daytime pulsed dose rate brachytherapy as a new treatment option for previously irradiated patients with recurrent oesophageal cancer. Br J Radiol 2005;78:236вҖ“241, PMID: 15730988.
11. Yamaguchi S, Ohguri T, Imada H, et al. Multimodal approaches including three-dimensional conformal re-irradiation for recurrent or persistent esophageal cancer: preliminary results. J Radiat Res 2011;52:812вҖ“820, PMID: 22020080.
12. Nonoshita T, Sasaki T, Hirata H, et al. High-dose-rate brachytherapy for previously irradiated patients with recurrent esophageal cancer. Radiat Med 2007;25:373вҖ“377, PMID: 17952540.
13. Teli MA, Mushood GN, Zargar SA, Andrabi WH. Comparative evaluation between re-irradiation and demand endoscopic dilatation vs endoscopic dilatation alone in patients with recurrent/reactivated residual in-field esophageal malignancies. J Cancer Res Ther 2008;4:121вҖ“125, PMID: 18923204.
14. Russo JK, Rosen L. TomoTherapy stereotactic body radiation therapy (SBRT) for the salvage treatment of locally recurrent esophageal adenocarcinoma following trimodality therapy: a case report. Tumori 2011;97:406вҖ“410, PMID: 21789024.
15. Sharma V, Mahantshetty U, Dinshaw KA, Deshpande R, Sharma S. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2002;52:310вҖ“315, PMID: 11872275.
Fig.В 1Computed tomography images of at diagnosis (primary), at the time of recurrence, and treatment response of re-irradiation in three patients with tracheaesophageal fistula. 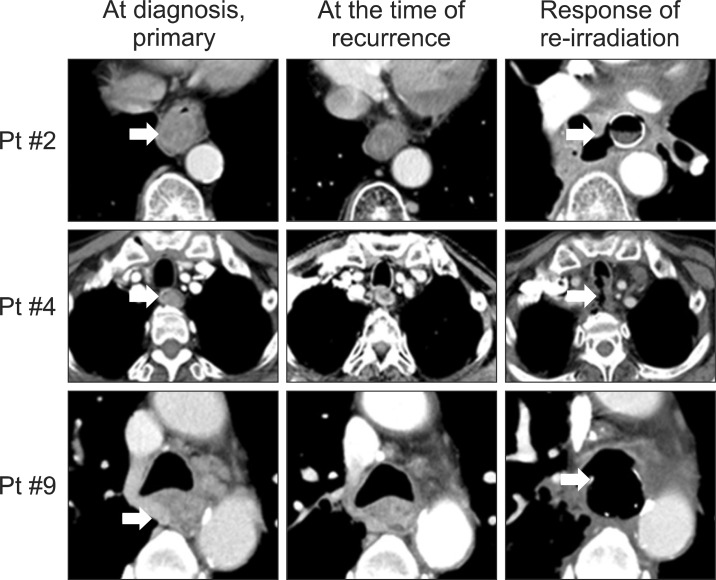
TableВ 1Patient characteristics at the time of primary radiotherapy 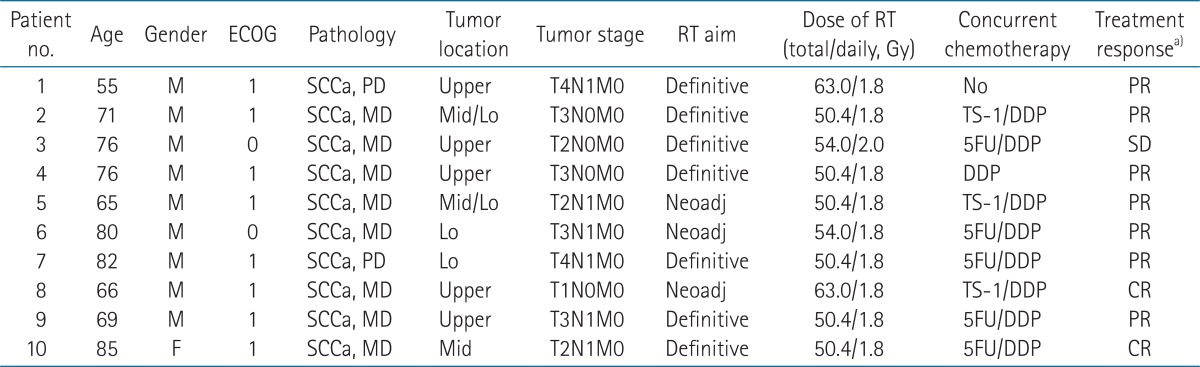 ECOG, Eastern Cooperative Oncology Group; RT, radiotherapy; SCCa, squamous cell carcinoma; PD, poorly differentiated; MD, moderately differentiated; Upper, upper thoracic esophagus; Mid, middle thoracic esophagus; Lo, lower thoracic esophagus; Neoadj, neoadjuvant; DDP, cisplatin; 5FU, fluorouracil; PR, partial response; SD, stable disease; CR, complete response. a)Three months later after end of primary RT. TableВ 2Patient characteristics at the time of re-irradiation, and treatment results of re-irradiation 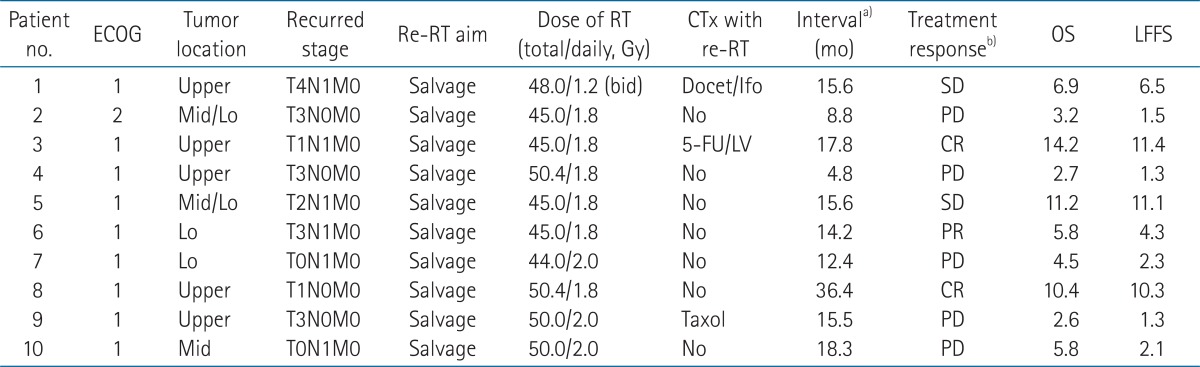 Upper, upper thoracic esophagus; Mid, middle thoracic esophagus; Lo, lower thoracic esophagus; Docet, docetaxel; Ifo, ifosfamide; FU, fluorouracil; LV, leucovorin; CTx, chemotherapy; re-RT, re-irradiation; OS, overall survival; LFFS, local failure-free survival. a)Time interval between initial irradiation and re-irradiation. b)Three months later after end of re-RT. TableВ 4Summary of re-irradiation (re-RT) of esophagus after primary definitive (concurrent chemo-) radiotherapy 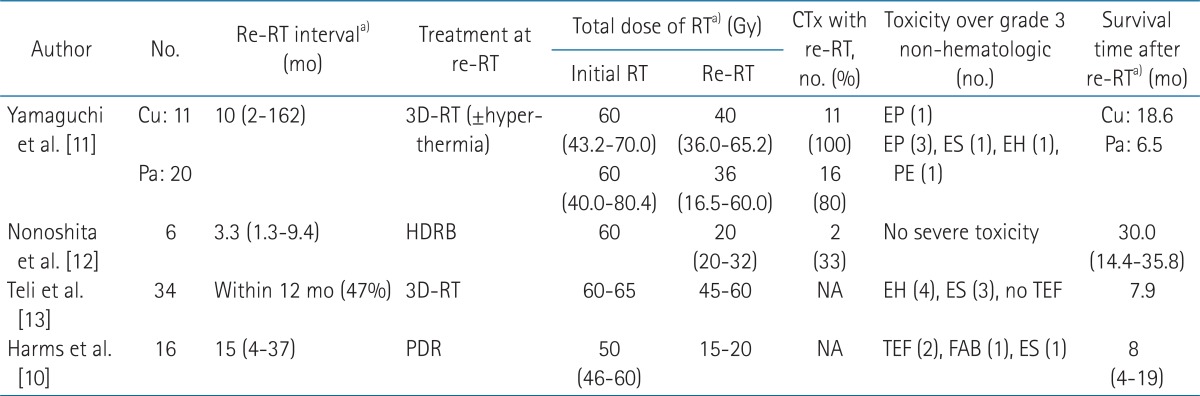 CTx, concurrent chemotherapy; Cu, curative group; Pa, palliative group; NA, not assessed; HDRB, high-dose-rate brachytherapy; PDR, pulsed dose rate brachytherapy; EP, esophageal perforation; ES, esophageal stricture; EH, esophageal hemorrhage; PE, pericardial effusion; TEF, tracheoesophageal fistula; FAB, fatal arterial bleeding. a)Values are presented as median (range). |
|
||||||||||||||||||||||||||||||||||||||||||||

 |
 |