Parotid gland sparing effect by computed tomography-based modified lower field margin in whole brain radiotherapy
Article information
Abstract
Purpose
Parotid gland can be considered as a risk organ in whole brain radiotherapy (WBRT). The purpose of this study is to evaluate the parotid gland sparing effect of computed tomography (CT)-based WBRT compared to 2-dimensional plan with conventional field margin.
Materials and Methods
From January 2008 to April 2011, 53 patients underwent WBRT using CT-based simulation. Bilateral two-field arrangement was used and the prescribed dose was 30 Gy in 10 fractions. We compared the parotid dose between 2 radiotherapy plans using different lower field margins: conventional field to the lower level of the atlas (CF) and modified field fitted to the brain tissue (MF).
Results
Averages of mean parotid dose of the 2 protocols with CF and MF were 17.4 Gy and 8.7 Gy, respectively (p < 0.001). Mean parotid dose of both glands ≥20 Gy were observed in 15 (28.3%) for CF and in 0 (0.0%) for MF. The whole brain percentage volumes receiving >98% of prescribed dose were 99.7% for CF and 99.5% for MF.
Conclusion
Compared to WBRT with CF, CT-based lower field margin modification is a simple and effective technique for sparing the parotid gland, while providing similar dose coverage of the whole brain.
Introduction
Whole brain radiotherapy (WBRT) has been implemented as one of the effective radiotherapy techniques for palliative therapy in the patients with multiple brain metastases [1]. Prophylactic cranial irradiation, which has been established as standard therapy showing survival benefit in the patients with small-cell lung cancer [2,3], is also performed with the WBRT technique. In defining the conventional WBRT field margin, lenses and the aero-digestive tract have been regarded as risk organs, but the parotid gland has not been a concern. However, through computed tomography (CT)-based simulation, we have found that a significant volume of parotid glands is included in the conventional WBRT field margin and have suggested that the parotid gland should be regarded as a risk organ according to the parotid doses used for WBRT [4]. Although xerostomia after WBRT is rarely encountered in clinical practice, the potential risk of it should be considered according to the parotid gland dose, especially in patients with long-term expected survival.
Many institutions have already modified the WBRT field margin, especially in the area of parotid glands with confinement to the brain tissue, allowing for a better sparing of parotid glands. However, the effect of these modifications has not been well described and evaluated. Therefore, we conducted a comparison study between the WBRT plans using different lower field margins: 1) conventional field to the lower level of the atlas (CF), which has been used conventionally in our institution, and 2) modified field fitted to the brain tissue in the area of parotid glands (MF). Herein, we present the differences in the dose-volume statistics of the parotid glands and normal tissue complication probabilities (NTCPs) for xerostomia between 2 WBRT plans.
Materials and Methods
From January 2008 to April 2011, 53 patients were treated with WBRT in our institution, using CT-based simulation and enrolled into the study; an additional 32 patients from a previously published study that analyzed dose-volume statistics of the parotid gland were enrolled as well [4]. This study was approved by the institutional review board of our institution. All patients received WBRT for the palliation of central nervous system (CNS) symptoms caused by brain metastasis or a primary brain tumor. The primary tumors from which brain metastases occurred included the following: 34 lung cancers, 8 breast cancers, 8 gastrointestinal tumors, 2 brain tumors, and 1 bladder cancer. Thirty-two patients (60.4%) were male and the patients ranged in age from 28 to 80 years (median, 60 years).
For CT-based simulation, all patients were immobilized with thermoplastic masks in the supine position. CT images were obtained using a Philips Big Bore Brilliance CT (Philips Medical System, Eindhoven, The Netherlands), including whole brain and cervical spines with 5 mm slice intervals. We defined a clinical target volume (CTV) as whole brain tissue from the vertex to foramen magnum. Whole brain volume was delineated using the auto-segmentation wizard function of the Varian Eclipse external beam planning system ver. 7.1 (Varian Medical Systems, Palo Alto, CA, USA). The planning target volume (PTV) was expanded symmetrically from CTV with a 1 cm margin except a caudal margin of 0.5 cm. Parotid glands were contoured on non-contrast enhanced CT images, slice by slice.
We planned 2 WBRT plans using both CF including lower level of atlas (Fig. 1A) and MF for sparing the parotid glands (Fig. 1B). For the MF planning of parotid sparing, the multileaf collimator (MLC) was fitted with 5 mm in the area of parotid glands and the other area was followed the margin of CF. The prescribed dose was 30 Gy in 10 fractions at the isocenter of 6 MV bilateral beams regardless of the field modification. For dose calculation, we used the Varian Eclipse external beam planning system ver. 7.1.
According to these data, we compared the dose-volume statistics between the CF and MF plans. Whole brain dose coverage was estimated using percentage volume of the CTV >98% and >105% of the prescribed dose. We compared the dose-volume statistics of parotid glands and the NTCPs for xerostomia, using the Lyman-Burman-Kutcher model [5]. We calculated the NTCPs with parameter sets from 3 published studies of head and neck radiotherapy [6-8].
Survival time was defined as the interval between day of CT-simulation and the day of death or last follow-up. The paired t-test was used to compare the differences between CF and MF plans. All statistical analyses were performed with SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
Results
The median survival time of the 53 patients was 6 months with a follow-up period of 1-24 months. In Fig. 2, dose-volume histograms (DVHs) of one patient (Fig. 2A) and parotid glands of conventional and modified plans in 53 patients (Fig. 2B) are illustrated. Mean parotid volumes were 21.1 cm3 (range, 8.5 to 43.3 cm3) for the right gland and 22.1 cm3 (range, 8.0 to 40.1 cm3) for the left gland. Median whole brain volume (CTV) was 1,327.6 cm3 and ranged from 1,108.4 to 1,571.0 cm3.
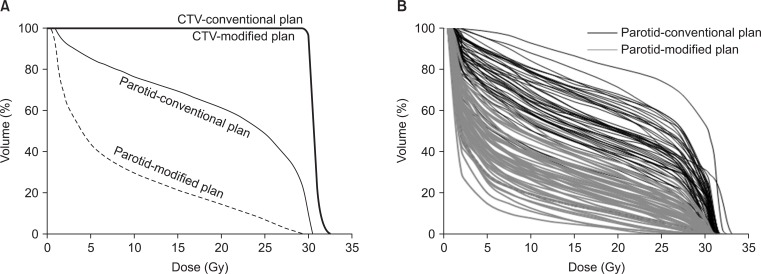
Dose-volume histograms of conventional and modified field. (A) Parotid glands and whole brain (clinical target volume, CTV). (B) Parotid glands in in 53 patients.
Dose-volume statistics of parotid gland are summarized in Table 1. Percentage volumes receiving >20 Gy (V20) and 30 Gy (V30) for parotid glands were significantly different between CF and MF plans (V20: 48.4% vs. 18.2%, p < 0.001; V30: 14.5% vs. 1.5%, p < 0.001). Averages of mean values for parotid doses were 17.4 Gy (range, 10.5 to 26.2 Gy) in CF plans and 8.7 Gy (range, 3.2 to 14.8 Gy) in MF plans (p < 0.001).
Mean values of percentage volume of the CTV receiving >98% of prescribed dose were 99.7% (range, 97.6% to 100.0%) for the CF plan and 99.5% (range, 97.3% to 100.0%) for the MF plan (Table 2). Percentage volume of the CTV receiving >105% of prescribed dose ranged from 2.4% to 46.8% (mean, 20.9) for the CF plan and from 2.6% to 45.6% (mean, 20.9) for the MF plan.
Of 106 individual glands, mean parotid doses ≥20 Gy were observed in 29 (27.4%) for CF protocols and 0 (0.0%) for MF protocols. The numbers of patients with a mean parotid dose of both glands ≥20 Gy were 15 (28.3%) for CF plans and 0 (0.0%) for MF protocols, respectively. There were no patients with a mean parotid dose ≥25 Gy except for 1 patient with CF plans, with mean parotid dose of 26.2 Gy. Compared to the CF plans, MF plans showed a significant improvement in NTCPs for xerostomia, regardless of the parameter sets (Table 3).
Discussion and Conclusion
In this study, we evaluated the parotid sparing effect of CT-based plan implementing the modified lower field margin fitted to the brain tissue compared to the conventional field in WBRT and showed a dosimetric improvement of the MF plan in parotid sparing. Mean parotid doses of both glands significantly decreased after modifying the lower field margin (mean: CF vs. MF, 17.4 vs. 8.7 Gy; p < 0.001). The number of patients with a mean parotid dose for both glands ≥20 Gy were 15 (23.8%) in CF plans. In contrast, there was no patient with a mean parotid dose ≥20 Gy in MF plans. Based on the analysis of parotid gland dose, WBRT using the CF plan can lead to a considerably higher parotid dose level (mean dose, ≥20 Gy) which may induce salivary flow reduction [9]. However, our results showed that the WBRT technique with CT-based modified lower field margin significantly reduced the parotid dose to less than the recommended dose limit, preventing parotid dysfunction (at least one parotid dose <20 Gy or mean dose of both glands <25 Gy) [10]. The values of NTCP using parameter sets from head and neck cancer showed significant reduction in the MF protocol (Table 1). The NTCP of severe xerostomia or flow reduction >75% of the normal level ranged from 0.00 to 0.67 (averages: NTCPEmami = 0.00, NTCPEisbruch = 0.07, NTCPRoesink = 0.13) in the CF technique. However, the range of NTCP in the MF technique was from 0.00 to 0.11 (averages: NTCPEmami = 0.00, NTCPEisbruch = 0.00, NTCPRoesink = 0.04). Although these NTCP estimations may be different from the settings of WBRT, these results suggest the parotid sparing effect of a CT-based MF technique.
The CT-based modification or reduction of lower field margin did not compromise the whole brain volume (CTV) dose coverage. The percentage volumes of the CTV receiving >98% of prescribed dose between CF and MF plans were 99.7% and 99.5%, respectively (p < 0.001). These results were statistically significant, but were not clinically significant. In both protocols, the percentage volume of the CTV receiving >105% of prescribed dose was the same (20.9%) (Table 2). These target coverage data showed that the DVH curve was not affected by the modified lower field margin (Fig. 2A). We determined the PTV by expanding the CTV with a caudal margin percentage of 5 mm, which is generally accepted PTV margin in radiotherapy of brain tumor. However, the caution should be taken to apply the 5 mm PTV margin in the patients expecting the intrafractional movement.
Although the CT-based modified lower field margin did not compromise the target dose coverage, the conventional field fitted to the lower level of cervical spine is clinically practical. In the era of 2-dimensional radiotherapy, modified field margin intersecting the body of cervical spine can make it difficult to design a radiation field for subsequent palliative radiotherapy of metastasis developed at just below the margin. However, palliative radiotherapy to the marginal area of previous radiation field is almost possible, while sparing the spinal cord within dose limits using CT-based radiotherapy.
There is no worldwide consensus in defining the lower field margin, which mainly affect the parotid dose in WBRT. In this study, CF plan with margin of lower level of atlas may be inappropriate to use as a baseline conventional plan for comparing the dose of parotid gland. However, the generous margin has been used in conventional 2-dimensional WBRT, even up to the level of C2 spine [11,12]. Because the level of the lower atlas can be considered as one of less generous margin, CF plan in this study may represent one of the conventional WBRT, which has neglect the parotid gland dose.
Modern intensity modulated radiotherapy (IMRT) techniques, including volumetric arc therapy, can be used to improve dose homogeneity and to spare complex shaped risk organs, such as the hippocampus [11-14]. To spare the parotid gland in WBRT for the patients expecting long-term survival, IMRT can be used just as in the case of head and neck radiotherapy. However, it is not cost-effective to adopt all WBRT cases, because most patients expect short-term survival. In this context, the use of a CT-based modified lower filed margin is a technically simple and effective method for sparing the parotid gland compared to IMRT.
The limitation of the MF technique is that it is not suitable for patients with combined upper cervical spine metastasis or leptomeningeal seeding, in which the lower margin can be extended to the lower cervical spine. In these cases, a more precise technique, such as IMRT, can be considered to spare the parotid gland.
The actual risk of xerostomia may be low even in the subpopulation with higher parotid doses. The level of the mean parotid dose in this study (maximum, 26.2 Gy in CF protocol) is sufficient to reach complete recovery of the preradiotherapy salivary function according to the prospective follow-up study describing the parotid gland recovery after radiotherapy [15]. To our best knowledge, xerostomia caused by WBRT had not been reported in the literature. However, abrupt salivary dysfunction can be followed by WBRT [9]. In the patients receiving less than 26 Gy of mean parotid dose, the relative decrease of salivary flow rate at 6-12 months after radiotherapy is about 50% of the level of pre-radiotherapy [15]. Acute parotitis presenting fever, dry mouth, pain, swelling, and tenderness can be observed after WBRT [16]. These acute phase symptoms can diminish the quality of life and may be more meaningful than that of chronic phase in the patients with a short expected survival. The potential risk of chronic xerostomia should also be considered in patients with long terms of expected survival even after WBRT. In the future, the clinical significance of the dose to the parotid in WBRT should be evaluated in a well-designed prospective study.
In conclusion, compared to conventional WBRT, CT-based lower field margin modification is a simple and effective method for sparing the parotid gland with similar dose coverage of whole brain.
Acknowledgments
This work was supported by the new faculty research fund of Ajou University School of Medicine.
Notes
No potential conflict of interest relevant to this article was reported.