Introduction
A considerable proportion of patients with early-stage non-small cell lung cancer (NSCLC) are unable or unwilling to undergo surgery, usually due to the presence of combined diseases or advanced age. The treatment of choice for such patients has usually been conventional radiotherapy (RT) using radiation doses of approximately 60 to 70 Gy delivered over 6 to 7 weeks. However, treatment outcomes were unsatisfactory with local failure rates as high as 60% to 70% in some series [1].
Over the past decade, stereotactic body RT (SBRT) has gradually replaced conventional RT for medically inoperable patients with early-stage NSCLC [2]. SBRT is a high-precision RT technique that delivers a highly conformal and hypofractionated ablative radiation dose over a short period of time. Up to 60 Gy is delivered in SBRT in as few as three fractions within 1 week, with steep increases in the tumoricidal effect; 60 Gy in three fractions is presumed to be equal to as much as 150 Gy in conventional fractionation [2]. Several prospective trials of SBRT demonstrated impressive local control, exceeding 90% at 3 years, with acceptable toxicity for patients with inoperable early-stage NSCLC [3-5].
In lung SBRT, the technical aspects of RT including image guidance and motion management are of particular relevance. They critically depend upon precise target localization to deliver a high radiation dose per fraction, while the tumors move continuously due to respiration [6]. Four-dimensional computed tomography (4D CT) correlates respiratory motion with the CT acquisition process, providing patient-specific information about tumor size, shape, and position in different phases of the respiratory cycle. Patients breathe freely during CT acquisition, and the images are retrospectively sorted into 10 respiration-based bins [7].
To account for tumor motion, the International Commission on Radiation Units and Measurements Report 62 introduced the concept of an internal target volume (ITV), defined as the clinical target volume (CTV), plus an additional margin to account for geometric uncertainties due to variable tumor motion [8]. In 4D CT, an ideal method of determining ITV is accomplished by contouring the CTV on each of the 10 respiration phase image sets. However, this poses some challenges due to the 10-fold increased workload for radiation oncologists. The aim of this study was to evaluate the suitability of ITV determination based on either two phases (peak-inspiration and peak-expiration) or four phases (peak-inspiration, peak-expiration, and two intermediate phases) using the ideal 10-phase-based ITV as a reference.
Materials and Methods
1. Patients
Fifteen patients with stage I NSCLC who underwent 4D CT simulation for treatment planning and received curative SBRT from 2011 to 2012 were analyzed retrospectively. Inclusion criteria for SBRT were as follows: pathologically confirmed NSCLC; clinical stage T1-2N0M0 according to the American Joint Committee on Cancer staging manual, 7th edition; longest tumor diameter of <5 cm; and Eastern Cooperative Oncology Group performance scale score of ÔëĄ2. The median age was 77 years (range, 68 to 86 years), and 10 patients (71.4%) were male. Only patients who were considered to be inoperable due to poor medical condition or refusal to undergo surgery were offered SBRT. The treated tumor was required to be further than 2 cm in all directions from the proximal bronchial tree [3].
2. ITV determination
During the 4D CT simulation, patients were immobilized in the supine position with the arms above the head in a vacuum-bag restriction system (Vac-Lock; CIVCO Medical Solutions, Kalona, IA, USA). Respiration-correlated 4D CT scans were performed during uncoached, quiet respiration using a Real-time Position Management system (Varian Medical Systems, Palo Alto, CA, USA) and a 16-slice CT scanner (Brilliance CT Big Bore; Philips Medical Systems, Cleveland, OH, USA) with a 0.2-cm slice thickness. Intravenous contrast was used in all patients. Data were acquired for the duration of a full respiratory cycle. Each reconstructed image was assigned to a specific respiratory phase to collectively yield a set of 10 CT images, each of which reflected 10% of the respiratory cycle. The gross tumor volume was delineated on the CT image for each respiratory phase using the 'lung window' setting. No expansion was made to account for microscopic disease extent, and the CTV was equivalent to the gross tumor volume.
Three approaches were utilized to determine the ITVs: 1) contouring the CTV on each of the 10 respiratory phases of the 4D CT data set and combining these CTVs to produce ITV10Phases; 2) combining 4 CTVs on the 0% (peak-inspiration), 20% (mid-expiration), 50% (peak-expiration), and 70% (mid-inspiration) respiratory phases to produce ITV4Phases; and 3) combining only 2 CTVs on both extreme respiratory phases (0% and 50%) to produce ITV2Phases. The planning target volume (PTV) was created by adding a 0.5-cm isotropic set-up margin around the ITV.
3. Analysis
The ITV4Phases and ITV2Phases were evaluated against ITV10Phases by comparing the matching index (MI). Because ITV4Phases and ITV2Phases are completely enveloped by ITV10Phases, the MI value of ITV4Phases and ITV2Phases was defined as the ratio of ITV4Phases and ITV2Phases, respectively, to the ITV10Phases. The maximum value of the MI is 1 if the two volumes are identical. The extent of tumor movement was expressed as the tumor motion index (TMI), defined by the ratio of ITV10Phases to CTVmean. The CTVmean was calculated as the mean value of 10 CTVs each delineated on 10 respiratory phases. Intergroup comparisons were conducted using the paired or independent-sample t-test, depending on the nature of the data. The correlation between TMI and MI, CTVmean, or pulmonary function parameters was analyzed using Spearman correlation coefficient. The level of statistical significance was set at p < 0.05; all reported p-values were two-tailed. Statistical analyses were performed using SPSS software ver. 14.0 (SPSS Inc., Chicago, IL, USA).
Results
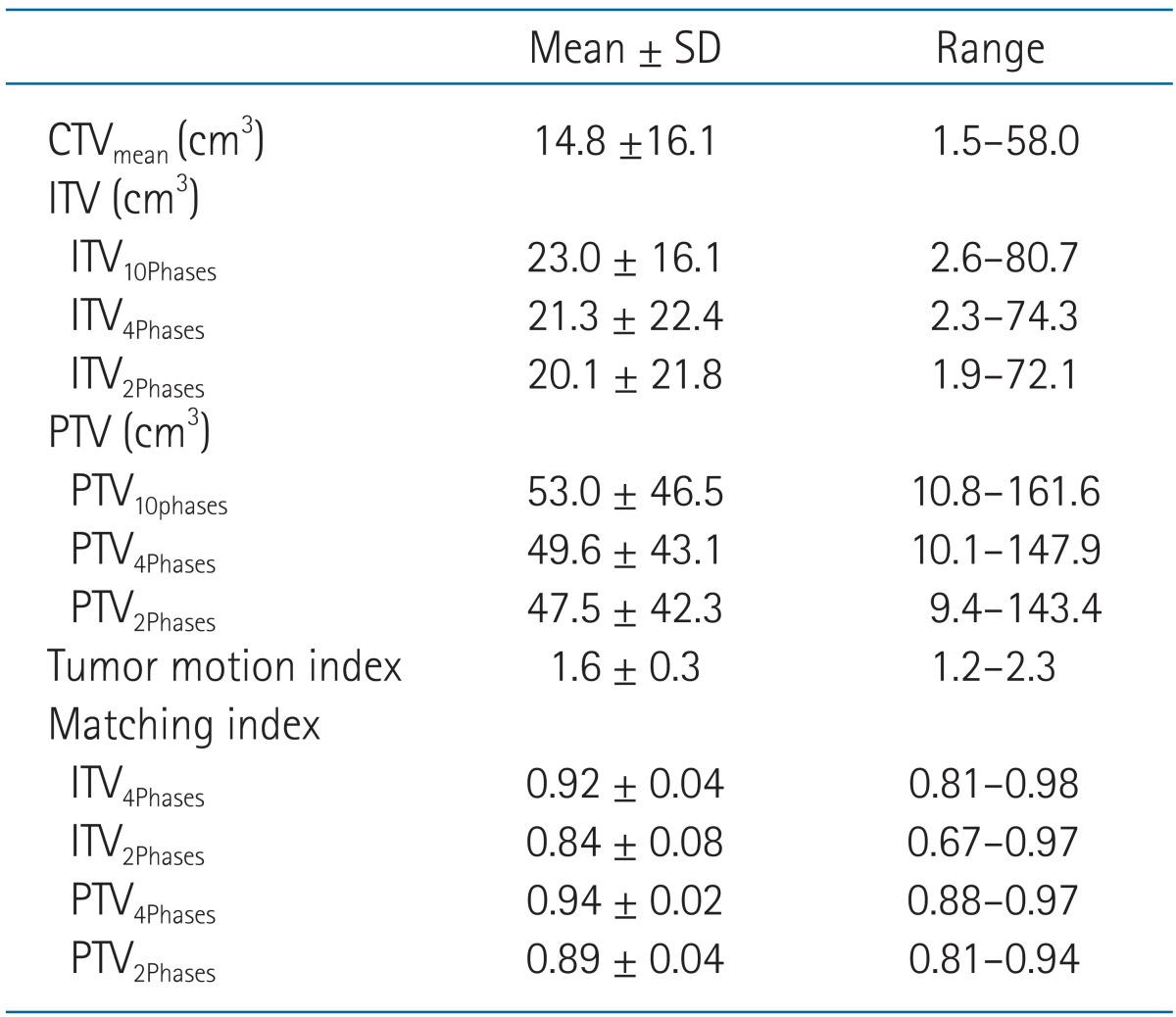
Table 1 shows the CTV, ITV, PTV, TMI, and MI values of all patients. The tumor was located in the upper, middle, and lower lobe in 7, 1, and 7 patients, respectively. The tumors located in lower lobe showed higher TMI, but with no statistical significance (lower lobe 1.7 ┬▒ 0.3 vs. mid-upper lobe 1.6 ┬▒ 0.3; p = 0.502). Similarly, the tumors having smaller size (CTVmean) showed higher TMI, but with no statistical significance (r = -0.304, p = 0.271). The pulmonary function data before SBRT were available in 12 patients, and the percentage predicted value of forced expiratory volume at 1 second (FEV1) and forced vital capacity (FVC) ranged 32%-134% (median, 81%) and 52%-142% (median, 88%), respectively. Patients with higher FEV1 or FVC showed higher TMI, but with no statistical significance (FEV1: r = 0.482, p = 0.133; FVC: r = 0.319, p = 0.339).
The ITV10Phases, ITV4Phases, and ITV2Phases were 23.0 ┬▒ 16.1, 21.3 ┬▒ 22.4, and 20.1 ┬▒ 21.8 cm3, respectively. There were significant differences between each ITV: 1) ITV10Phases vs. ITV4Phases, p = 0.002; 2) ITV10Phases vs. ITV2Phases, p = 0.001; and 3) ITV4Phases vs. ITV2Phases, p < 0.001. The MI of ITV4Phases (0.92 ┬▒ 0.04) was significantly higher than that of ITV2Phases (0.84 ┬▒ 0.08, p < 0.001). Also, the MI of PTV4Phases (0.94 ┬▒ 0.02) was significantly higher than that of PTV2Phases (0.89 ┬▒ 0.04, p < 0.001).
The MI of ITV4Phases was significantly correlated with TMI (r = -0.650, p = 0.009) (Fig. 1). When patients were grouped according to a median value of TMI (1.58), the ITV4Phases (20.5 ┬▒ 22.7 cm3) differed significantly compared with ITV10Phases (22.3 ┬▒ 24.1 cm3) in a group of patients (n = 8) with high TMI (p = 0.013). In contrast, the ITV4Phases (22.2 ┬▒ 23.8 cm3) did not differ statistically compared with ITV10Phases (23.9 ┬▒ 25.9 cm3) in a group of patients (n = 7) with low TMI (p = 0.192). Regarding PTVs in this group with low TMI, the difference between PTV10Phases (54.9 ┬▒ 49.0 cm3) and PTV4Phases (51.5 ┬▒ 44.4 cm3) was further decreased statistically (p = 0.204). Regardless of TMI, the MIs of ITV4Phases and PTV4Phases were significantly higher than those of ITV2Phases and PTV2Phases, respectively.
Discussion and Conclusion
SBRT is a safe and effective alternative therapy for patients with medically inoperable early-stage NSCLC [3-5]. As additional evidence, we recently reported favorable clinical outcomes, including local tumor control and low toxicity [2]. However, many of the original experiences with lung SBRT used standard, population-based margins for consideration of internal target movements and did not specifically delineate tumor contours that encompassed the tumor trajectory over the entire breathing cycle [9]. Such population-based estimates may overestimate or underestimate the margin needed for a given patient because breathing characteristics are quite variable among patients [10]. Overestimation increases the volume of healthy tissues exposed to high radiation dose, while underestimation results in missed targets and thus a decrease in tumor control. It has been shown that factors, such as tumor size, location, and pulmonary function tests cannot reliably predict respiration-driven lung tumor motion for an individual patient [11,12]. Similarly, in the present study, tumor size and location were not related to the extent of lung tumor movement (TMI). There are three basic methods of using 4D CT to deliver SBRT: an ITV method that uses static fields encompassing tumor motion during normal breathing, a gating method that synchronizes tumor location and 'beam on' timing, and tracking methods that track the tumor with continuous therapy [13]. The ITV approach is the most straightforward and most commonly adopted technique of the three methods. It has the potential to determine patient-specific ITVs to ensure adequate tumor coverage while minimizing unnecessary radiation to healthy tissues [14].
The need to contour CTVs in 10 respiratory phases to define the ITV is a major drawback to the routine clinical use of 4D CT scans. To reduce the workload of contouring multiple target volumes in 4D CT, a post-processing tool is occasionally used, including maximum intensity projection (MIP) images. The MIP reflects the highest data value encountered along the viewing ray for each pixel of volumetric data, displaying the brightest object along each ray [15]. However, Ezhil et al. [10] showed that MIP-based ITV determination underestimates the 10-phase-based ITV to a greater degree than does the extreme 2-phase-based ITV. The authors presented potential sources of uncertainty/error in the MIP-based approach as follows: the MIP image may not fully display mobile structures if the adjacent structures have similar (or higher) densities, which is the case for lesions located near the mediastinum, diaphragm, liver, or chest wall, and the tumor spicula cannot be visualized on the MIP because of smearing of the tumor edge. For the extreme 2-phase-based approach, tumor deformation between the two phases of breathing and the curved motion pathway during each breathing cycle may introduce uncertainty. They cautioned against adopting a method of ITV determination using MIP or an extreme 2-phase-based approach, which can miss a significant amount of tumor volume [10]. The present study also showed that the ITV2Phases significantly underestimated ITV10Phases, even in patients with low TMI.
In a phantom study using a single-slice CT scanner, Jin et al. [16] showed that adequate estimation of ITV could be achieved by the addition of motion information of an intermediate phase to that of the extremes of motion, especially in cases of small tumors with large longitudinal motion. Using multislice 4D CT on patients, the present study investigated ITV4Phases, which included two intermediate respiratory phases (mid-inspiration and mid-expiration) in addition to two extreme phases. By this additive workload, the MI could be significantly increased compared with that of ITV2Phases, and a statistically significant underestimation of ITV compared with the reference ITV10Phases disappeared in a group of patients with low TMI. Intermediate respiration phases, in addition to extreme phases, may be required to cope with lung motion uncertainty, such as nonlinearity and hysteresis induced by physiologic effects during free breathing [17,18]. A significant inverse correlation was found between the TMI and the MI of ITV4Phases (Fig. 1). Although the definitions of TMI and MI differed, Hof et al. [19] similarly reported that 4D CT-based PTV diverged from conventional CT-based PTV as the lung tumor motion increased. Abdominal compression may be helpful in reliably replacing ITV10Phases with ITV4Phases for more patients, especially those identified as showing a high TMI [20].
The present study had some limitations. First, the selection of patients with low TMI could be accomplished after contouring CTV on all 10 respiratory phases. To apply ITV4Phases clinically, more practical methods to discern patients with less tumor motion should be explored. Examples may include the use of fluoroscopy simulator or the visual evaluation of peak-to-peak tumor motion using 4D CT images in the RT planning computer. Second, the number of analyzed patients was small and the MIP method was not used in this study. A further study including more patients and comparative analysis of MIP data will be necessary.
In conclusion, compared with the optimal but highly time-consuming ITV10Phases, ITV determination based on four respiratory phases, including two extreme and two intermediate respiratory phases, did not significantly underestimate the tumor volume of the selected patients with low TMI while it reduced the workload by more than half. If ITV determination by ITV10Phases is beyond the scope of routine clinical use, the ITV4Phases method rather than ITV2Phases may be an efficient alternative approach in SBRT for patients with early-stage NSCLC.