 |
 |
AbstractA clear consensus has not been established regarding the best treatment for solitary bone metastasis. Here, we reviewed the medical records of patients with a controlled primary malignancy who had only solitary spine metastasis without metastasis to the extraspinal bone or viscera and underwent treatment between April 2007 and December 2012 with stereotactic body radiosurgery using CyberKnife, with a total dose of 24 Gy in three to four fractions. During that time, there were only four cases. This was effective in each case, and all the four patients had no local failure and remained alive at a median follow-up of 68 months (range, 64 to 80 months). Although our experience is limited, this study suggests that stereotactic body radiotherapy could be a feasible, safe, effective, and noninvasive alternative treatment for solitary spine metastasis in patients who are medically inoperable or unsuitable for surgery.
IntroductionMetastatic disease in the skeleton is common, affecting up to 70% of cancer patients. A true isolated bone metastasis is rare however, with an incidence of only 2% to 3% among cases in which skeletal spread is noted, with the vertebral column being the most frequent site of bony metastases [1]. Most patients are asymptomatic, although when symptoms do occur they usually take the form of persistent bone pain [2]. A clear consensus has not been established for the treatment for solitary bone metastases, which is the most challenging to validate.
Case ReportsThe treatment summary for all the patients is presented in Table 1 and 2. The patients were treated with a total dose of 24 Gy in three to four fractions over 3 or 4 days. We encompassed radiologically visible tumors seen on computed tomographic (CT) scans with a 2- or 3-mm margin as the treatment volume. Spinal cord/cauda equina volume was based on the CT images fused with magnetic resonance images (MRIs) and consistently defined as the volume extending from 6 mm above to 6 mm below the radiosurgical target.
A conformal inverse planning technique and iterative optimization algorithms were used. The dose delivered to the spinal cord was kept at <21.4 Gy in three fractions, and the spinal cord constraint was 10 Gy to a 10% volume of the spinal cord. A limit of 2,100 cGy was set as the maximum dose to the esophagus and small bowel to avoid toxicity. The median isodose value covering the treatment volume was 74% (range, 73% to 78%) of the prescribed dose. Images of the patients are shown in Figs. 1-4.
1. Case 1A 54-year-old man was diagnosed with renal cell carcinoma for which he underwent a nephrectomy in 2006, confirmed as pT3N0M0, stage III, without adjuvant therapy. Two years later a bone metastasis was detected in the T8 spine (Fig. 1), and the patient complained of severe back pain. His verbal/visual analogue scale (VAS) score was 8, and his Eastern Cooperative Oncology Group (ECOG) performance status was 1. We recommended vertebroplasty before radiosurgery because of a pathologic compression fracture in T8 spine, but the patient refused this. Radiosurgery was administered instantly, with a total dose of 24 Gy in 3 fractions, after which, the patient's pain improved with a VAS score of 3 and he required less than half the maximum dose of analgesics. Treatment with the oral chemotherapeutic agent, sunitinib has been continued. He was followed for 36 months until July 2011 without any evidence of recurrence or metastasis on positron emission tomographic-computed tomographic (PET-CT) and MRI scans. He was confirmed to be alive until November 2013, but we had no current information about him from the past 2 years because he subsequently transferred to a hospital near his home.
2. Case 2In 2006, a 58-year-old man was diagnosed with hepatocellular carcinoma, cT1N0M0, stage I in segment 8 and was treated with transcatheter arterial chemoembolization and radiofrequency ablation, which controlled the liver lesion. Two years later, he visited hospital because of upper back and right shoulder pain. This was investigated by performing PET-CT and CT, which revealed a T3 spinal metastasis (Fig. 2). The patient was referred to our hospital for radiosurgery. At admission, he complained of sensory changes in both legs and in the lower abdominal area, and weakness in both lower extremities with fourth/fifth muscle strength. His VAS score for pain was 7, even with opioid analgesics. At that time, his ECOG performance status was 2, and the level of serum α-fetoprotein was elevated at 702 ng/mm3.
A spine MRI before radiosurgery revealed a marrow infiltrative lesion at the pedicle, articular processes, laminas, and spinous process of T3, findings compatible with bone metastasis. Seven days after radiosurgery, the patient reported that his pain had started to improve. Subsequently, he reported that he was free of pain and no longer needed analgesics. Two weeks after radiosurgery, his neurologic symptoms resolved and his serum α-fetoprotein level dropped to 36.31 ng/mm3, and after 4 months, this level normalized at 2.99 ng/mm3. Since the radiosurgery was performed on the T3 spine, no further treatment was given until April 2012. At 52 months after radiosurgery, two liver lesions (8 and 12 mm) were found on segments 3 and 6, respectively, and treated with radiofrequency ablation. These two lesions were verified as controlled on follow-up liver CT and PET-CT and evidenced by a serum α-fetoprotein level of 2.4 ng/mm3. The metastatic T3 lesion had been radiologically controlled for 71 months after radiosurgery without relapse, pain, or neurological symptoms; in fact, there had been no evidence of disease.
3. Case 3A 56-year-old woman patient was treated with modified radical mastectomy followed by chemoradiation therapy for breast cancer, pT2N2M0, stage IIIA in 1996. Nine years after primary treatment, a bone metastasis was detected in the T9 and T10 spine. Palliative radiotherapy was administered with a total dose of 30 Gy over 12 fractions to the T8-T11 spine, and palliative chemotherapy was continued.
The patient returned to hospital in 2007 because of back pain. At presentation, she complained of pain with VAS score of 5, without neurologic symptoms and an ECOG performance status of 1. MRI revealed bone metastasis on the left side of the vertebral body, pedicle, lamina, both articular processes of T10 spine, and left articular process of T9 spine (Fig. 3). Radiosurgery was administered to the metastatic spine lesions at 6 Gy/fraction to a total dose of 24 Gy. The patient reported being in less pain within 7 days of radiosurgery and was subsequently pain-free and continued to be so until the last follow-up. She did not receive further chemotherapy or hormonal therapy until 74 months after the radiosurgery. In May 2013, a new lung metastasis was noted in the right upper lobe and treated with wedge resection. The histological findings were compatible with lung metastasis and the pathological report confirmed that the 3 × 2.2-cm tumor, visceral pleural invasion, and hormonal receptors were again negative. On follow-up PET-CT and chest CT at 80 months after the radiosurgery (6 months after the wedge resection), no fluorodeoxyglucose uptake was seen in the T9 and T10 spine and there were no abnormal lesions suggestive of recurrence or metastasis.
4. Case 4A 52-year-old man was diagnosed with rectal cancer, stage III (pT3N1M0) in 1999 and was treated with low anterior resection followed by chemoradiation therapy and then an oral chemotherapeutic agent. After 6 years, lung metastasis had developed in the left lower lobe and was treated with wedge resection followed by chemotherapy. After a further 2 years, a follow-up PET-CT showed a focal hypermetabolic lesion in the C3 vertebral body (Fig. 4), a finding compatible with metastasis, although this was asymptomatic. He was referred to our hospital for radiosurgery to the C3 spine as primary treatment.
After radiosurgery of 24 Gy over 3 fractions, no further chemotherapy was given, and no further tumor recurrence has been noted for 66 months.
Discussion and ConclusionInnovations in molecular imaging and advances in molecular oncology have meant that increasingly small lesions can be detected, and the unambiguous detection of truly solitary metastases is possible at an earlier stage. This in turn increases the likelihood that aggressive treatment by surgical excision, stereotactic body radiosurgery, targeted chemotherapy, or immunotherapy could eradicate the lesion.
En bloc spondylectomy in patients with solitary metastasis of the spine generally yields good local control. Tomi et al. [3] reported a median survival of more than 38 months and local failure in only 2 of 28 patients treated with en bloc resection. Similarly, Sakaura et al. [4] reported local recurrence in 2 of 12 patients, 23 and 25 months after surgery, and that only 5 patients died of the disease. In a study reported by Melcher et al. [5], none of the 12 patients had local recurrence at a mean follow-up of 32 months and 4 patients developed distant metastasis, of whom, 2 died of lung metastasis. Sundaresan et al. [6] demonstrated median survival time of 30 months and local failure in one of 6 patients (17%).
In patients with spinal instability that manifested as a pathologic fracture, progressive deformity, or neurologic deficit, and had clinically significant neural compression by bone or bone debris, surgical management should be considered as an initial treatment. Lesions unlikely to respond to radiation or chemotherapy, such as renal cell carcinoma, should also be considered as candidates for operative intervention. Also, surgical management of spine metastasis could be performed to prevent or relieve pain and to improve overall quality of life.
However, surgical management for a spine metastasis may not be indicated in patients considered medically inoperable or in patients who refuse invasive techniques. In such cases, the use of noninvasive modalities such as stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT) are preferred. These are advanced approaches for the delivery of a single (SRS) or a few (SBRT) large doses of radiation [7]. They can facilitate delivery of an optimal therapeutic dose to a lesion with a rapid dose fall-off near the spinal cord, resulting in potentially better local control and long-term survival in select patients. Recent studies based on imaging and clinical symptoms showed that the rate of local tumor control ranged from 77% to 100% when using these techniques [8]. In the largest prospective study from the University of Pittsburgh, 500 spinal metastases in 393 patients were treated with single-fraction radiosurgery up to a mean dose of 20 Gy (range, 12.5 to 25 Gy) [9]. Pain relief was achieved in 86% of cases on long-term follow-up (median, 21 months) without radiation-induced spinal cord toxicity and the rate of overall radiologic tumor control was 88% of all cases. At the MD Anderson Cancer Center by Chang et al. [10], a phase I/II study of 74 spinal lesions in 63 patients treated with 3 or 5 fraction of 27 to 30 Gy reported that the actuarial 1-year tumor progression-free incidence was 84% for all patients with the median follow-up of 21.3 months. Yamada et al. [11] at the Memorial Sloan-Kettering Cancer Center reported on 103 lesions in previous unirradiated 93 patients treated with radiosurgery. At a median follow-up of 15 months, actuarial local control was 90% with a 45-month actuarial survival of 36%.
The majority of these studies report on oligometastases of the spine or on spine lesions that occurred spontaneously with metastases to the extraspinal bone or viscera. There is therefore limited information available concerning solitary spine metastasis. A prospective study at the Henry Ford Hospital examined 61 lesions in 49 radiation-naive patients. Treatment consisted of a single fraction of 10 to 16 Gy to the single spine lesion, and resulted in pain relief in 85% of cases with a median duration of 13 months, and a 1-year overall survival rate of 74% [12]. Our results show effective local control and long-term survival for a solitary spine metastasis treated with a total dose of 24 Gy in three to four fractions. Here, we observed no local failure with complete pain relief of 75% and a median disease-free survival of 59 months (range, 36 to 74 months), although one patient had not been followed up for the most recent 2 years. All the four patients remained alive after a median follow-up of 68 months (range, 64 to 80 months). There have been no cases of severe toxicity to date. We also found no newly developed or progressive pathologic fractures in the treated spine after radiosurgery and no radiation-induced myelopathy has occurred. The low failure rate in our study may be because of both the delivery of a tumor curable radiation dose and the biologically favorable primary malignancy. Primary malignancy types can be ranked according to growth as slow (breast, rectum, and prostate), moderate (kidney, liver, and uterus), and rapid growth (lung and stomach) [13,14]. The primary tumor sites of the 4 patients in our study were the breast, rectum, kidney, and liver. All these are classified as having slow and moderate growth, associated with a good prognosis.
Radiation therapy for spinal metastasis has been proven to be effective in reducing pain around 70%-80% [15]. There have been multiple prospective randomized trials comparing the rate of pain relief with palliative radiation therapy according to different total dose and fractionation scheme. These trials have shown pain relief equivalency for dosing schema, including 30 Gy in 10 fractions, 26 Gy in 6 fractions, 20 Gy in 5 fractions, and a single 8 Gy fraction [16,17]. SBRT can be delivered in a short treatment time (i.e., several days) and so is convenient. SBRT could offer potentially better local control due to the large doses of radiation delivered. So, concerning these strengths, we suggest that SBRT could be considered in patients who required noninvasive modality for solitary spine metastasis.
Long-term survival outcomes in patients treated with SBRT for solitary spine metastasis were comparable to those of patients who underwent en bloc spondylectomy. Our limited experience suggests that SBRT may be an attractive option for the treatment of solitary spine metastasis, as all four patients remained alive without local failure at a median follow-up of 68 months (range, 64 to 80 months). SBRT is therefore a potentially feasible, safe, effective, and noninvasive alternative treatment for solitary spine metastasis in patients who are medically inoperable or unsuitable for surgery.
References1. Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin Radiat Oncol 2006;16:120–130, PMID: 16564447.
2. Pakos EE, Gartzonikas DN, Tsekeris PG, Xenakis TA. Solitary tibial osteolytic lesion. Case Rep Med 2009;2009:352085PMID: 19718252.
3. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306, PMID: 11224867.
4. Sakaura H, Hosono N, Mukai Y, Ishii T, Yonenobu K, Yoshikawa H. Outcome of total en bloc spondylectomy for solitary metastasis of the thoracolumbar spine. J Spinal Disord Tech 2004;17:297–300, PMID: 15280758.
5. Melcher I, Disch AC, Khodadadyan-Klostermann C, et al. Primary malignant bone tumors and solitary metastases of the thoracolumbar spine: results by management with total en bloc spondylectomy. Eur Spine J 2007;16:1193–1202, PMID: 17252218.
6. Sundaresan N, Rothman A, Manhart K, Kelliher K. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976) 2002;27:1802–1806, PMID: 12195075.
7. Chawla S, Abu-Aita R, Philip A, Lundquist T, Okunieff P, Milano MT. Stereotactic radiosurgery for spinal metastases: case report and review of treatment options. Bone 2009;45:817–821, PMID: 19540375.
8. Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys 2008;71:652–665, PMID: 18514775.
9. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32:193–199, PMID: 17224814.
10. Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 2007;7:151–160, PMID: 17688054.
11. Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 2008;71:484–490, PMID: 18234445.
12. Ryu S, Jin R, Jin JY, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage 2008;35:292–298, PMID: 18215498.
13. Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990;15:1110–1113, PMID: 1702559.
14. Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J 2010;19:215–222, PMID: 20039084.
15. Wu JS, Wong R, Johnston M, Bezjak A, Whelan T. Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 2003;55:594–605, PMID: 12573746.
16. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol 2007;25:1423–1436, PMID: 17416863.
17. Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965–976, PMID: 21277118.
Fig. 1Images of case 1 patient. (A) Pretreatment magnetic resonance image (MRI), (B) pretreatment computed tomography (CT), (C) sagittal MRI image, (D) positron emission tomography-CT at 13 months after radiosurgery, and (E) the dose distribution of radiosurgery. 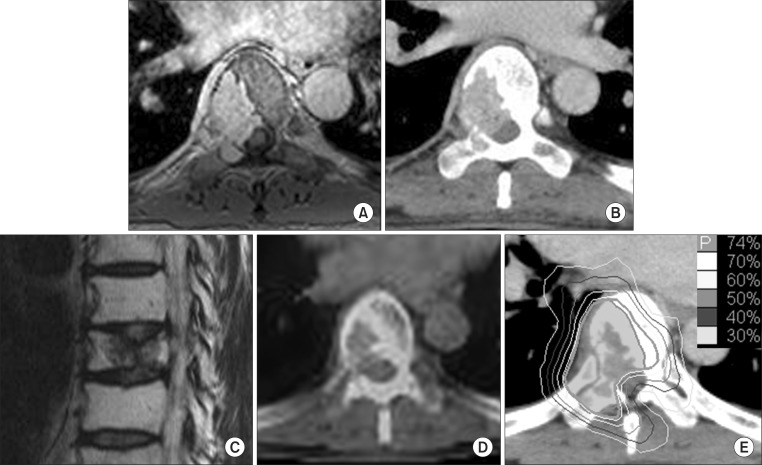
Fig. 2Images of case 2 patient. (A, B) Pretreatment computed tomography (CT), (C) pretreatment positron emission tomography (PET)-CT, (D) PET-CT at 12 months after radiosurgery, and (E) the dose distribution of radiosurgery. 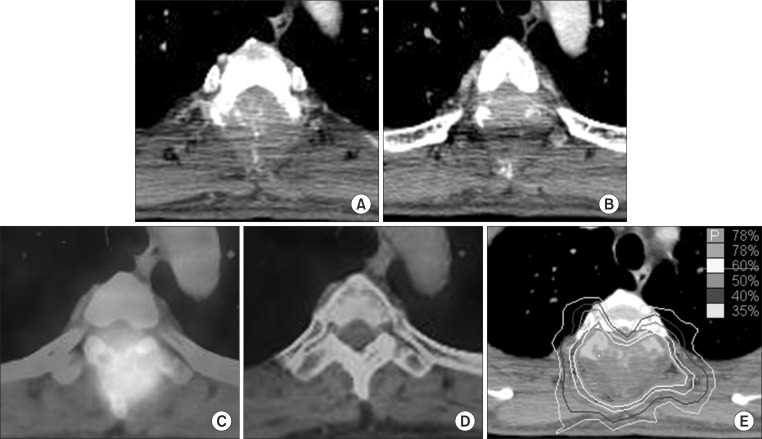
Fig. 3Images of case 3 patient. (A, B) On axial and (C) sagittal magnetic resonance images (MRIs) of pretreatment, (D) positron emission tomography-computed tomography at 12 months after radiosurgery, and (E) the dose distribution of radiosurgery. 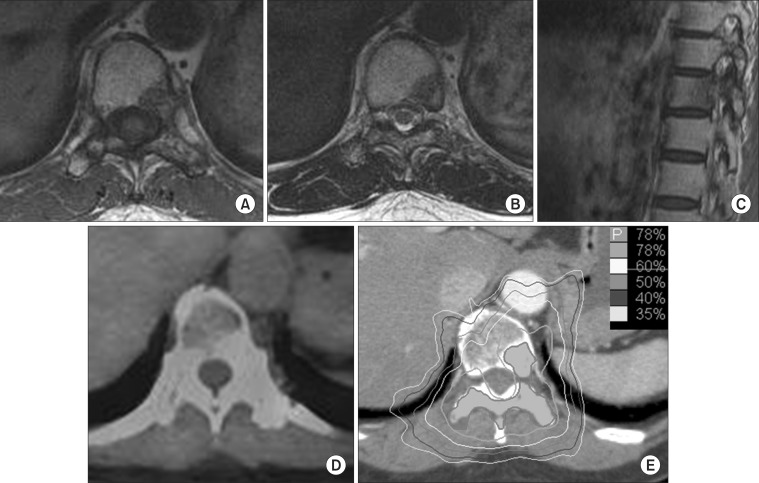
Fig. 4Images of case 4 patient. (A) Pretreatment computed tomography (CT), (B) pretreatment positron emission tomography (PET)-CT, (C) PET-CT at 9 months after radiosurgery, and (D) the dose distribution of radiosurgery. 
|
|
||||||||||||||||||||||||||||||||||||

 |
 |