 |
 |
AbstractPurposeWe designed this study to identify and suggest the reasonable timing of adjuvant radiotherapy in the treatment of uterine carcinosarcoma according to the surgical intent and patterns of progression.
Materials and MethodsWe retrospectively analyzed a total of 50 carcinosarcoma patients diagnosed between 1995 and 2010. Among these 50 patients, 32 underwent curative surgery and 13 underwent maximal tumor debulking surgery. The remaining five patients underwent biopsy only. Twenty-six patients received chemotherapy, and 15 patients received adjuvant radiotherapy.
ResultsThe median follow-up period was 17.3 months. Curative resection (p < 0.001) and stage (p < 0.001) were statistically significant factors affecting survival. During follow-up, 30 patients showed progression. Among these, eight patients (16.0%) had loco-regional progression only. The patients who had received adjuvant radiotherapy did not show loco-regional progression, and radiotherapy was a significant negative risk factor for loco-regional progression (p = 0.01). The time to loco-regional progression was much earlier for non-curative than curative resection (range, 0.7 to 7.6 months vs. 7.5 to 39.0 months).
IntroductionCarcinosarcomas, also known as malignant mixed Müllerian tumors, are complex tumors displaying histological features of both carcinoma and sarcoma [1]. Primary carcinosarcoma can arise anywhere along the female genital tract, but the uterus is the most common site of origin [2]. However, the real incidence of carcinosarcoma only accounts for less than 5% of all uterine carcinoma cases, with an estimated annual incidence of less than two per 100,000 women [3]. Nevertheless, it is still an important tumor due to its unique features such as its carcinomatous and sarcomatous characteristics and its poor prognosis [4].
Because of the relatively low incidence of uterine carcinosarcoma, an independent staging classification system has not been assigned to this tumor. Since 1988, uterine sarcomas have been staged by the International Federation of Gynecologists and Obstetricians (FIGO) via the same surgical staging criteria utilized for uterine endometrial carcinoma [5]. In addition, standard endometrial cancer treatment is used to treat patients with carcinosarcoma [6]. However, there are clear pathologic and clinical differences between these two tumors, suggesting that it may be necessary to modify the treatment for carcinosarcoma.
Though the typical treatment for uterine carcinosarcoma is surgery [6], several recent reports have suggested that radiotherapy could improve local control compared to surgery alone [7-9]. While there is still some controversy regarding the impact of radiotherapy on overall survival, adjuvant radiotherapy is an important modality in carcinosarcoma treatment [10]. Nevertheless, the appropriate timing of radiotherapy after surgery has not yet been determined.
We conducted the present study in order to identify and suggest the reasonable timing of adjuvant radiotherapy in the patients with uterine carcinosarcoma based on the surgical intent and patterns of progression.
Materials and MethodsWe retrospectively collected the medical record of patients who had been diagnosed with carcinosarcoma between January 1995 and December 2010 from the gynecologic oncology tumor board registry of the institution. Pathologic confirmation of all carcinosarcoma specimens was conducted by a gynecologic pathologist. Clinical and pathological data were entered into a prospective database from January 1995 to December 2010. Staging of the disease was performed according to the FIGO 2009 staging criteria.
During the time period analyzed, a total of 50 patients had been treated at the institute. The median age of the patients was 60 years (range, 35 to 87 years). The primary site was cervical carcinosarcoma in four patients and the primary site could not been determined in one patient. Four patients had a performance status score of 2 or more by the Eastern Cooperative Oncology Group (ECOG) guidelines. The descriptive characteristics of the patients are shown in Table 1.
Surgical procedure with curative intent was performed in 32 patients (64.0%), and maximal tumor debulking surgery was performed in the other 13 patients (26.0%). All 45 patients underwent extra-fascial hysterectomy. In the remaining five patients (10.0%), only biopsy was performed. Using the FIGO 2009 staging criteria, ten patients were stage IA, ten patients were stage IB, and two patents were stage IIB. All 22 patients received curative resection (extra-fascial hysterectomy and bilateral salphingo-ophorectomy in 21 patients and left salphingo-ophorectomy in one patient). Only ten patients out of sixteen patients with staged IIIC received curative resection, other five with stage IIIC and eight of 12 with stage IVB received maximal debulking surgery, and the others biopsy only.
Almost half of the patients (26, 52.0%) received chemotherapy. Chemotherapy was not usually given to stage I patients, but two patients who had near complete myometrial tumor invasion received chemotherapy. Chemotherapy was not used in four stage III or IV patients who had poor performance status and/or old age or who were reluctant to receive further treatment. Chemotherapy regimens varied among single or combination agents, including ifosfamide, cisplatin, adriamycin, epirubicin, and/or paclitaxel. However, most of the patients (19 patients) received a combination of ifosfamide and cisplatin.
Adjuvant radiotherapy was performed in 15 patients (30.0%). Only three patients who were stage III received radiotherapy. The median interval between surgery and radiotherapy was 30 days (range, 20 to 130 days). One patient who had received biopsy only was treated with concurrent chemoradiotherapy. The whole pelvis was irradiated using the four-field box technique to 50.4 Gy at 1.8 Gy per fraction using 15 MV photon beams. Following staging using the 2009 FIGO stage criteria, intracavitary brachytherapy (30 Gy/6 fractions) was used for two stage I patients.
To identify the pattern of tumor progression, we evaluated the first site of progression after surgery. All progression was diagnosed by either clinical or radiological examination. We defined loco-regional progression as progression at the stump of the vagina and/or the pelvic lymph node that was targeted in adjuvant whole pelvic radiotherapy. We considered as loco-regional progression if local symptom had aggravated and/or definite increment of tumor size had showed on imaging studies in patients received biopsy only. The other patterns of progression were divided into para-aortic lymph node, intraperitoneal (without solid organ involvement), and distant metastasis. If the progression was detected more than one site in a month, we categorized it as the more advanced pattern.
The chi-square test or Fisher's exact test were used to compare the other clinical variables and loco-regional progression. Progression-free survival (PFS) was calculated from the date of surgery to the date of event recognition or to the date of the final follow-up visit. Overall survival (OS) was measured from the date of surgery to the date of death or to the date of the final follow-up visit. Survival rates were compared for statistical significance (p-value less than 0.05) using log-rank analysis. PFS and OS were estimated using the Kaplan-Meier product-limit method. Univariate logistic regression analysis was used to evaluate the association between survival and various parameters. To evaluate the relationships between survival and various parameters using multivariate analysis, a stepwise procedure was performed using a logistic regression model including all the variables that attained or had a trend toward univariate statistical significance. A p-value of <0.05 was considered statistically significant. All calculations were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
Results1. Related diseaseFive of the 50 patients had been treated with tamoxifen due to breast cancer before a diagnosis of carcinosarcoma. The median interval between breast cancer surgery and carcinosarcoma diagnosis was 120 months (range, 32 to 140 months). Two patients also received whole breast radiotherapy.
Nine patients had history of prior pelvic radiotherapy (5 patients, rectal cancer; 3 patients, cervical cancer; 1 patients, dysgerminoma), and the range of the interval between radiotherapy and carcinosarcoma diagnosis was 73 to 181 months (median, 118 months). Other combined diseases were pituitary adenoma in 3 patients, hepatocellular carcinoma in 1 patient, and bronchioloalveolar carcinoma of the lung in 1 patient.
2. Survival rate and prognostic factorsThe median follow-up period was 17.3 months. Survival curves of all patients are displayed in Fig. 1 (PFS 1A and OS 1B). The relationships between survival and various parameters are summarized in Tables 2 (PFS) and 3 (OS). ECOG performance status (p < 0.001 in PFS, p = 0.001 in OS), curative resection (p < 0.001, both), stage (p < 0.001, both) were statistically significant factors on univariate analysis. In the stage-by-stage analysis, surgical intent was also found to be a significant related factor. However, the presence of related disease (p = 0.208 in PFS, p = 0.189 in OS) or previous pelvic radiotherapy (p = 0.471 in PFS, p = 0.290 in OS) were not related with survival outcome. Although adjuvant chemotherapy (p < 0.001 in PFS, p = 0.012 in OS) was negative prognostic factor in survival, this result probably related with advanced stages. Therefore, in the multivariate analysis, curative resection (p < 0.001) and stage (p < 0.001) were significantly related to PFS and OS.
3. Stage-by-stage comparison of survival with endometrial cancerThe endometrial cancer survival rate was referenced from the FIGO 26th annual report [11]. Survival curves according to FIGO stage are shown in Fig. 2. The survival rate of patients with stage I or II carcinosarcomas was not grossly different from that of equivalent stages of endometrial cancer, despite there being too few cases to make an exact comparison (Fig. 2A). However, survival of patients of stage IIIC showed a considerable difference between carcinosarcoma and endometrial cancer. The 1- and 2-year survival rates were 53.3% and 33.3%, respectively, in carcinosarcoma, and 89.9% and 74.5% in endometrial cancer (Fig. 2B).
4. Patterns of disease progressionDuring the follow-up period, 30 patients (60.0%) showed progression, and the median time to progression from surgery was 11.3 months (range, 0.4 to 162.2 months). Most progressions (24/30, 80%) occurred within 1 year after surgery. Table 4 shows the first progression sites for these patients according to the surgical intent and FIGO staging system. The patterns of first progression were loco-regional in eight patients (16.0%, stump 6 and pelvic lymph node 2), para-aortic lymph node (and/or loco-regional) in six patients (12.0%), intra-peritoneal in eight patients (16.0%), and distant in eight patients (16.0%, lung, 4; bone, 2; supraclavicular, 1; multiple, 1). Loco-regional progression was the main progression pattern in the four patients received biopsy only (two patients had also intraperitoneal progression). The other one patient treated with concurrent chemoradiotherapy had intraperitoneal progression. Two patients received surgery only and four patients treated with surgery and chemotherapy progressed loco-regionally. Loco-regional progression was not detected in patient who received adjuvant radiotherapy.
5. Factors related to loco-regional progression and duration after surgeryA total of 8 patients experienced loco-regional progression after surgery and/or adjuvant chemotherapy. Among these patients, none had received adjuvant radiotherapy. Radiotherapy was a significant negative risk factor for loco-regional progression (p = 0.01). Surgical intent (p = 0.002) and depth of invasion (p = 0.018) were significant factors related to loco-regional progression. After curative resection, the median time to loco-regional tumor progression was 10.0 months (range, 7.5 to 39.0 months), compared to 2.3 months after maximal debulking surgery (range, 0.7 to 7.6 months), and only 1.1 months after biopsy (range, 0.9 to 1.9 months). Among the patients who received biopsy only, one patient received concurrent chemoradiotherapy, and that patient did not progress loco-regionally for more than 8 months.
Discussion and ConclusionAlthough carcinosarcomas are composed of epithelial and mesenchymal elements, it is currently thought of as an 'undifferentiated' or 'metaplastic' carcinoma rather than a uterine sarcoma [12]. Therefore, we recommend that carcinosarcoma be treated according to the National Comprehensive Cancer Network (NCCN) guidelines for epithelial carcinoma [10]. However, carcinosarcomas do have distinctive clinical and pathologic features separate from those of endometrial carcinomas, which might necessitate modification of the treatment of carcinosarcoma. Despite many recent advances, the optimal treatment of these patients remains controversial [6,13].
The prognosis of patients with stage I to III carcinosarcoma remains worse than those with uterine carcinoma, with 5-year survival rates ranging from 33% to 39% [7,14]. Also in this study, advanced stage (III or IV) carcinosarcoma showed poorer survival than endometrial carcinoma in a stage-by-stage comparison.
The mainstay of current uterine carcinosarcoma surgery is total hysterectomy and bilateral salpingo-oopherectomy; however, the standard surgical extent of this disease has not yet been clearly identified [14]. In a retrospective analysis of the records for 1,855 patients with stage I to III carcinosarcoma from the Surveillance, Epidemiology, and End Results (SEER) database, lymph node dissection and the number of dissected lymph nodes were associated with overall survival [6]. Furthermore, a recent retrospective study found that cytoreductive surgery with a goal of achieving complete gross resection was associated with an improvement in OS among advanced stage carcinosarcoma patients [15]. This result was in accordance with the results of the present study, with curative resection patients living longer than 5 years compared to less than 6 months in the other patients. Furthermore, despite the small number of cases analyzed, patients who had received only biopsy showed significantly shorter survival than patients who received maximal debulking and curative resection. To maximize survival outcomes in the treatment of carcinosarcoma, the goal of the surgery should be a complete gross resection.
As discussed above, the principal treatment for uterine carcinosarcoma is surgery, but the high rates of both local and distant relapse after surgery have demonstrated the need for effective adjuvant treatment [7]. Historically, ifosfamide has been the most effective chemotherapy agent [16,17]. Recently, the Gynocologic Oncology Group (GOG) reported that the use of cisplatin plus ifosfamide chemotherapy compared favorably over whole abdomino-pelvic radiation for adjuvant therapy in all stages of carcinosarcoma [18]. In the present study, most of the patients who received chemotherapy were treated with the combination of cisplatin and ifosfamide. The finding of our study that adjuvant chemotherapy was not related to survival is likely due to the small number of cases analyzed. The results of a recent phase II GOG trial suggested that a combination of paclitaxel and carboplatin is also a tolerable and effective regimen [19].
Because a large series study is not possible due to the low incidence of carcinosarcoma, it is very important that all clinical experiences be evaluated for various prognostic factors and treatment results through comparison with other series. This study was carried out with this aim, and we retrospectively evaluated the interval between surgery and radiotherapy in uterine carcinosarcoma patients in order to determine the optimal interval.
Even though there are still some conflicting results related to overall survival, adjuvant radiotherapy has been shown to improve local control in patients with carcinosarcoma [7-9]. In addition, in the present study, none of the patients who received adjuvant radiotherapy showed loco-regional progression. In contrast, three of the 17 patients who underwent curative resection without radiotherapy showed loco-regional progression, and the time to loco-regional progression was longer than 6 months after curative resection. Therefore, if a patient receives curative resection and are indicated adjuvant chemotherapy, adjuvant radiotherapy might be delayed during chemotherapy. However, loco-regional progression proceeded rapidly in non-curatively resected patients, with interval times as short as less than one month. We think adjuvant radiotherapy should be considered in those patients as soon as possible after healing of the surgical wound, despite there is a possibility that the biology of the disease itself is too aggressive to control loco-regionally by the adjuvant radiotherapy. Despite only one case, the patient who received concurrent chemoradiotherapy after biopsy was loco-regional progression-free for more than 7 months. As in other studies, we think the effectiveness of radiotherapy in the loco-regional control of carcinosarcoma was verified in this study [7-9].
To our knowledge, this report is the first to evaluate the optimal interval between surgery and adjuvant radiotherapy in uterine carcinosarcoma patients. Our findings might be helpful to determine when adjuvant radiotherapy should be delivered in patients with carcinosarcoma for the purpose reducing loco-regional progression.
The present study did have some limitations. First, retrospective data may be impossible to confirm and may conceal selection biases. Second, due to the retrospective study design, cases of present study were very small and had heterogeneous treatment parameters. Despite these limitations, the present study provides important information regarding the optimal timing of adjuvant radiotherapy in carcinosarcoma. Additional large-scale studies will be needed in order to obtain more accurate results.
References1. Robinson-Bennett B, Belch RZ, Han AC. Loss of p16 in recurrent malignant mixed mullerian tumors of the uterus. Int J Gynecol Cancer 2006;16:1354–1357, PMID: 16803529.
2. Teo SY, Babagbemi KT, Peters HE, Mortele KJ. Primary malignant mixed mullerian tumor of the uterus: findings on sonography, CT, and gadolinium-enhanced MRI. AJR Am J Roentgenol 2008;191:278–283, PMID: 18562759.
3. Nielsen SN, Podratz KC, Scheithauer BW, O'Brien PC. Clinicopathologic analysis of uterine malignant mixed mullerian tumors. Gynecol Oncol 1989;34:372–378, PMID: 2548936.
4. Silverberg SG, Major FJ, Blessing JA, et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus: a Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol 1990;9:1–19, PMID: 2152890.
5. Pradhan TS, Stevens EE, Ablavsky M, Salame G, Lee YC, Abulafia O. FIGO staging for carcinosarcoma: can the revised staging system predict overall survival? Gynecol Oncol 2011;123:221–224, PMID: 21889193.
6. Nemani D, Mitra N, Guo M, Lin L. Assessing the effects of lymphadenectomy and radiation therapy in patients with uterine carcinosarcoma: a SEER analysis. Gynecol Oncol 2008;111:82–88, PMID: 18674808.
7. Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed mullerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys 2004;58:786–796, PMID: 14967435.
8. Chi DS, Mychalczak B, Saigo PE, Rescigno J, Brown CL. The role of whole-pelvic irradiation in the treatment of early-stage uterine carcinosarcoma. Gynecol Oncol 1997;65:493–498, PMID: 9190981.
9. Gerszten K, Faul C, Kounelis S, Huang Q, Kelley J, Jones MW. The impact of adjuvant radiotherapy on carcinosarcoma of the uterus. Gynecol Oncol 1998;68:8–13, PMID: 9454652.
10. National Comprehensive Cancer Network. Recent updates to NCCN clinical practice guidelines in oncology (NCCN guidelines) [Internet]. Fort Washington, PA: National Comprehensive Cancer Network; cited 2012 May 20. Available from: http://www.nccn.org/professionals/physician_gls/recently_updated.asp.
11. Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95(Suppl 1):S105–S143, PMID: 17161155.
12. McCluggage WG. Uterine carcinosarcomas (malignant mixed mullerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer 2002;12:687–690, PMID: 12445244.
13. Tinkler SD, Cowie VJ. Uterine sarcomas: a review of the Edinburgh experience from 1974 to 1992. Br J Radiol 1993;66:998–1001, PMID: 8281393.
14. Gadducci A, Romanini A. Adjuvant chemotherapy in early stage uterine sarcomas: an open question. Eur J Gynaecol Oncol 2001;22:352–357, PMID: 11766739.
15. Tanner EJ, Leitao MM Jr, Garg K, et al. The role of cytoreductive surgery for newly diagnosed advanced-stage uterine carcinosarcoma. Gynecol Oncol 2011;123:548–552, PMID: 21945551.
16. Homesley HD, Filiaci V, Markman M, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:526–531, PMID: 17290061.
17. Sutton G, Brunetto VL, Kilgore L, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: a Gynecologic Oncology Group Study. Gynecol Oncol 2000;79:147–153, PMID: 11063636.
18. Wolfson AH, Brady MF, Rocereto T, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol 2007;107:177–185, PMID: 17822748.
19. Powell MA, Filiaci VL, Rose PG, et al. Phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: a Gynecologic Oncology Group study. J Clin Oncol 2010;28:2727–2731, PMID: 20421537.
Fig. 1Kaplan-Meier curves for progression-free survival rate (A) and overall survival rate (B) of carcinosarcoma patients after surgery or biopsy. 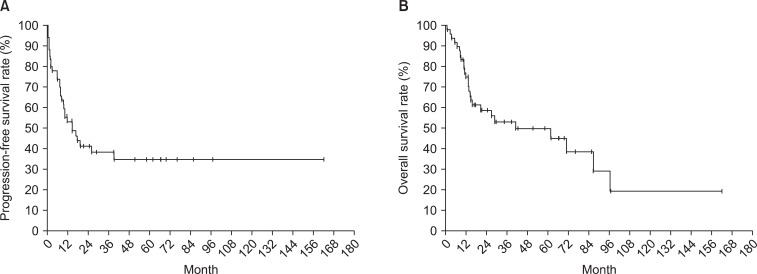
Fig. 2Kaplan-Meier curves for overall survival rate in patients with carcinosarcoma compared patients with endometrial (EM) carcinoma: stage-by-stage comparison. (A) Stage I-II, (B) stage III-IV. 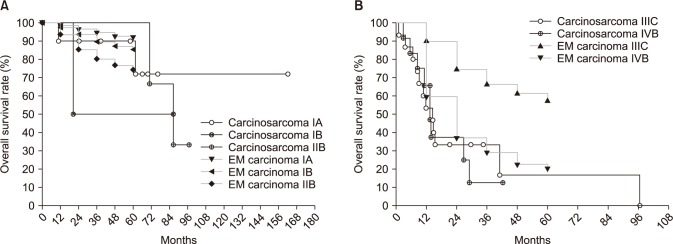
|
|
||||||||||||||||||||||||||||||||||||

 |
 |