Cardiac dose reduction with breathing adapted radiotherapy using self respiration monitoring system for left-sided breast cancer
Article information
Abstract
Purpose
To quantify the cardiac dose reduction during breathing adapted radiotherapy using Real-time Position Management (RPM) system in the treatment of left-sided breast cancer.
Materials and Methods
Twenty-two patients with left-sided breast cancer underwent CT scans during breathing maneuvers including free breathing (FB), deep inspiration breath-hold (DIBH), and end inspiration breath-hold (EIBH). The RPM system was used to monitor respiratory motion, and the in-house self respiration monitoring (SRM) system was used for visual feedback. For each scan, treatment plans were generated and dosimetric parameters from DIBH and EIBH plans were compared to those of FB plans.
Results
All patients completed CT scans with different breathing maneuvers. When compared with FB plans, DIBH plans demonstrated significant reductions in irradiated heart volume and the heart V25, with the relative reduction of 71% and 70%, respectively (p < 0.001). EIBH plans also resulted in significantly smaller irradiated heart volume and lower heart V25 than FB plans, with the relative reduction of 39% and 37%, respectively (p = 0.002). Despite of significant expansion of lung volume using inspiration breath-hold, there were no significant differences in left lung V25 among the three plans.
Conclusion
In comparison with FB, both DIBH and EIBH plans demonstrated a significant reduction of radiation dose to the heart. In the training course, SRM system was useful and effective in terms of positional reproducibility and patient compliance.
Introduction
Adjuvant radiation therapy after breast-conserving surgery is an essential treatment for early breast cancer [1]. Previous studies, however, have reported increased risk of radiation related toxicity resulting in non-breast cancer related deaths, which are mainly caused by cardiovascular disease and lung cancer [2,3,4]. Recently published study has reported that the cardiac mortality ratio for left sided (vs. right sided) breast cancer and the lung cancer mortality ratio for ipsilateral (vs. contralateral) breast cancer were raised during the third decade after radiation therapy [5]. Moreover, some agents widely used in breast cancer treatment, such as doxorubicin (Adriamycin) or trastuzumab (Herceptin) may induce cardiotoxic events [6,7]. Consequently, dose reduction to the heart and lungs is necessary to decrease the risk of radiation-related morbidity and possible mortality.
Various radiation techniques, e.g., breath-hold or respiratory gating and intensity-modulated radiation therapy (IMRT) [8], are proposed to reduce the dose to the heart and lungs. For left-sided breast cancer, a deep inspiration breath-hold (DIBH) technique is increasingly adopted to reduce the cardiac dose by displacing the heart out of the radiation field, and several studies have demonstrated its feasibility with promising clinical results [9,10,11,12,13,14,15]. An end inspiration gating (EIG) technique was also tried for the patients with unfavorable breath-holding capacity [10,16].
For respiration control, several devices, such as active breathing control (ABC) device, Real-time Position Management (RPM; Varian, Palo Alto, CA, USA) system, and Abches are tested for their clinical applicability [17]. RPM system has been widely used for respiratory gating radiotherapy especially for lung or liver cancer, and now installed in increasing number of institutions. The authors tried to use commercially available RPM system to monitor respiratory chest wall excursion during planning CT scan acquisition and real treatment, and developed an in-house self respiration monitoring (SRM) system. The patients can see their own graphical information of respiration cycle, its intensity and breath-hold time through the SRM system lying on the couch in the treatment position. This study was designed to quantify the benefit of breathing adapted radiotherapy techniques using RPM and SRM system for cardiac dose reduction by comparing the dose and volume parameters of CT scan data including free breathing (FB), DIBH, and end inspiration breath-hold (EIBH) for EIG treatment.
Materials and Methods
1. Patient selection, respiratory training, and CT simulation
This study involved 22 patients who had been referred for adjuvant radiation therapy after breast conserving surgery for early stage (≤T2 and N0) left-sided breast cancer. Between December 2012 and May 2013, patients were included consecutively if informed consent had been obtained. All patients were told about the purpose of this study, and agreed with acquisition of additional CT scanning. Of the 22 patients, 6 patients were treated with DIBH protocol and 16 patients received conventional FB treatment.
Before the acquisition of planning CT scan image, patients were introduced and trained for RPM and SRM system. Patients were positioned on the flat table in the treatment room with infrared reflecting marker block on the upper abdominal wall near the xiphoid process to detect breathing motion by RPM system. Using an infrared tracking camera and a reflective marker block, RPM system measures the patient's respiratory pattern and the range of motion and displays them in a waveform. The gating thresholds are set when the breast target is in the desired portion of the respiratory cycle. These thresholds determine when the gating system turns the treatment beam on and off. SRM system that was developed in our institution was located about 30 to 40 cm away from patient's face (Fig. 1B). SRM system consists of a LCD monitor mounted on the flexible arm and movable stand (Fig. 1A). By the aid of signal distributer, this LCD monitor receives the same signal that RPM system sends to the control room monitor, and displays the animated graph of the breathing amplitude detected by RPM system (Fig. 1C). With this visual feedback system, patients were able to monitor their own breathing motion as it was observed by physician in the control room. All patients were trained with easy to control their respiratory pattern using SRM system, and to remember the appropriate breath-hold level of DIBH and EIBH techniques.

Self respiration monitoring (SRM) system for visual feedback of patient's breathing motion: (A) LCD monitor mounted on the flexible arm and movable stand, (B) patient positioned on the inclined breast board with the SRM system, (C) the animated graph of the breathing amplitude detected by Real-time Position Management system.
After finishing about 15 minutes of training course, patients were positioned on the inclined breast board in the CT simulation room with the ipsilateral arm above the head as the same position of real treatment with marker block on the belly. Patients underwent CT scans with 5-mm slice thickness during different breathing maneuvers including FB, DIBH, and EIBH (Fig. 2). For breath-holding maneuver, the excursion depth detected by RPM system during scanning time was limited by the maximum acceptable movement of 3 mm using the upper and lower gated thresholds.

Respiratory patterns of one patient detected by Real-time Position Management system during computed tomography scans with different breathing maneuvers including deep inspiration breath-hold (A), end inspiration breath-hold (B), and free breathing for end inspiration gating treatment (C). Dashed lines indicate upper and lower gating thresholds with the maximum acceptable movement of 3 mm, and determine when the gating system turns the beam on and off.
2. Delineation of target and organs-at-risk
Target and organs-at-risk were delineated on each CT scan by the same physician. The tumor bed clinical target volume (CTV) was defined using surgical clips and/or associated seroma. The planning target volume (PTV) encompassed visible breast tissue including tumor bed CTV being limited by the outer contour of the rib, 5 mm from skin surface. The anatomic landmarks for contouring the PTV were midline (medial), anterior border of serratus anterior (lateral), inferior border of clavicle (superior), and 1 cm below inframammary fold (inferior).
The heart contour was defined as the ventricles, atria and auricles from the level of the root of the aorta and pulmonary trunk to the apex of the heart as described by Feng et al. [18]. The heart volume extended to the pericardium including myocardium and circulating blood volume. From the root of the aorta to the apex of the heart, left anterior descending (LAD) coronary artery was delineated if visible on non-contrast CT image, usually with diameters of 4 to 5 mm, by adopting the method of Taylor et al. [19]. In case that it was not possible to identify coronary artery directly, delineation of LAD was inferred by the location of the anterior interventricular groove [19]. The 1-cm radius of LAD was outlined by adding a radial margin to compensate the uncertainties generated by difficulty in identification and beating movement of the heart [19].
3. Treatment planning
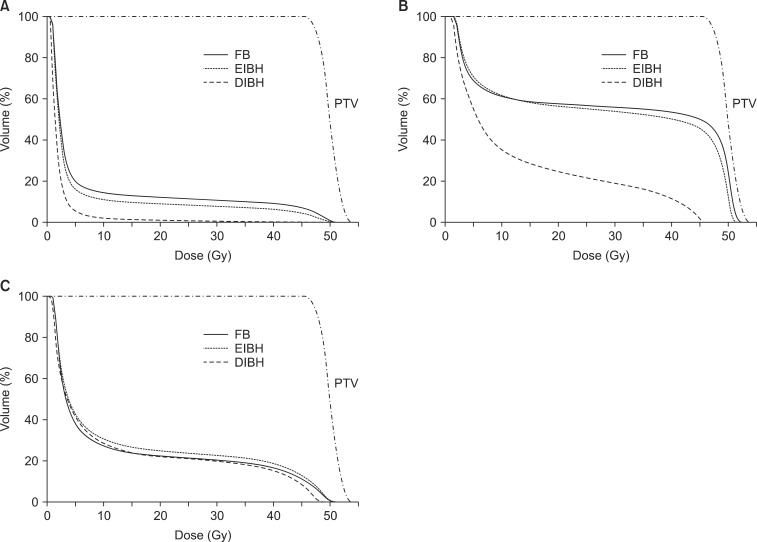
For each scan, treatment plans were generated by the Eclipse treatment planning system (ver. 10.0, Varian) with a pair of wedged tangential fields using 6 MV photon half-beams of the Varian Novalis Tx linear accelerator. The prescription to PTV was 50.4 Gy in 28 fractions. All plans were optimized to ensure that volume coverage was kept between 95% and 110% of the prescription dose for tumor bed CTV and between 90% and 110% for PTV. The irradiated heart volume was defined as the heart volume covered by tangential fields. Dose-volume histograms were generated for each structure to compare parameters according to different breathing maneuvers (Fig. 3). For the heart, mean heart dose, maximum heart dose, and normal tissue complication probability (NTCP) of cardiac mortality were calculated [20]. The relative structure volume receiving more than 25 Gy was calculated for the heart (Heart V25) and the lung (Lung V25).
4. Data analysis
Statistical analysis was performed using SPSS ver. 12 (SPSS Inc., Chicago, IL, USA). The paired t-test was used to compare the dosimetric parameters for FB plan versus DIBH and EIBH plans. A two-tailed p < 0.05 was considered statistically significant.
Results
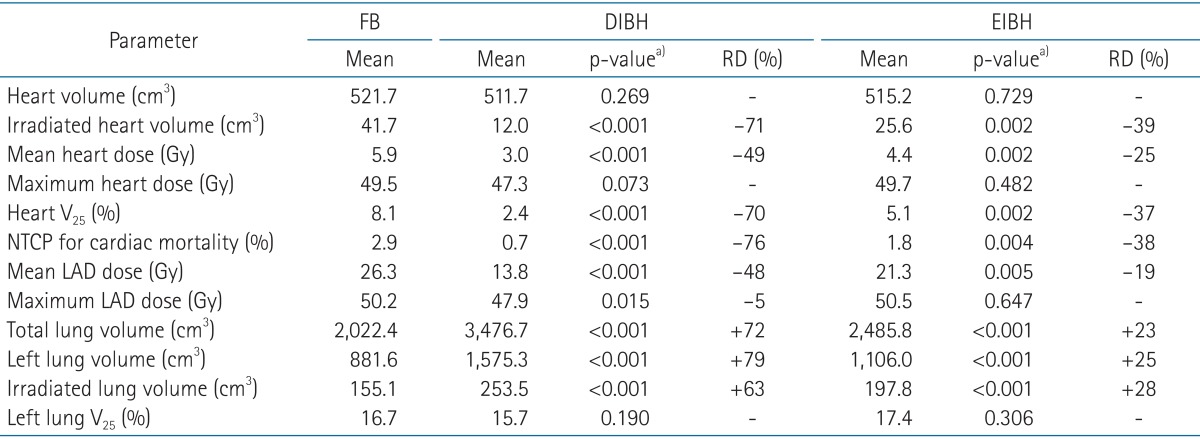
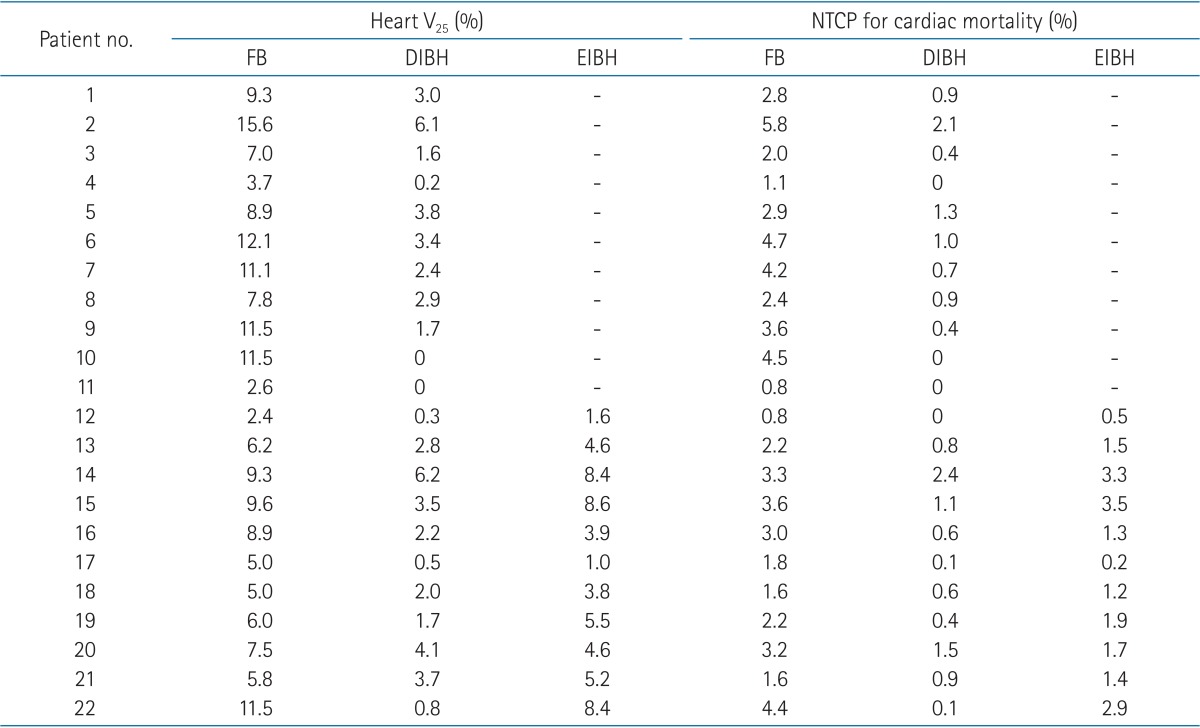
The median patient age was 48 years (range, 30 to 69 years). All patients completed CT scans with two different breathing maneuvers (FB and DIBH), and 11 of 22 patients underwent additional CT scan with EIBH technique. As shown in Table 1, total lung volume and left lung volume of CT scans with DIBH and EIBH were significantly larger than those of FB scan (p < 0.001), indicating that all patients successfully achieved the accurate breath-hold level. The average total lung volume of FB was 2,022.4 cm3, and those of DIBH and EIBH scan were 3,476.7 cm3 and 2,485.8 cm3, respectively. There was no significant difference in heart volumes among the three breathing maneuvers. The average heart volumes of FB, DIBH, and EIBH scan were 521.7, 511.7, and 515.2 cm3, respectively. When compared with FB plans, DIBH plans demonstrated significant reductions in irradiated heart volume, mean heart dose, and the heart V25 (Fig. 4A), with the relative reduction rate of 71%, 49%, and 70%, respectively (p < 0.001). EIBH plans also resulted in significantly smaller irradiated heart volume, lower mean heart dose, and the heart V25 than FB plans, with the relative reduction of 39%, 25%, and 37%, respectively (p = 0.002). Scatter plot of heart parameter showed that all data plotted under solid line indicating relative reduction in heart V25 of DIBH and EIBH plans in comparison with FB plans (Fig. 4A). Moreover, 86% of DIBH data located under dashed line indicating the 50% relative reduction.
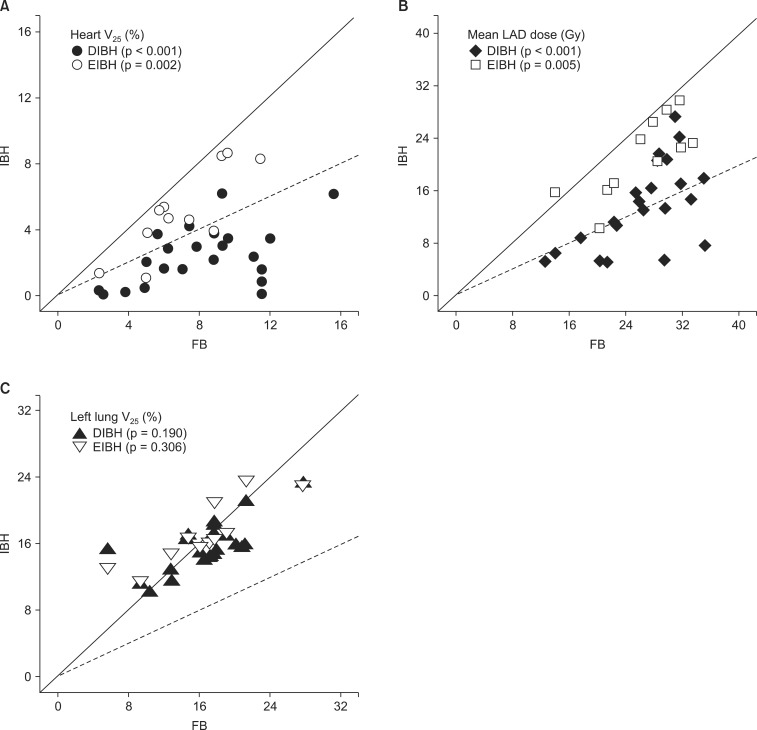
Scatter plots of dosimetric parameters for deep inspiration breath-hold (DIBH) and end inspiration breath-hold (EIBH) plans in comparison with free breathing (FB) plans; individual data under solid line means relative reduction of dosimetric parameter using inspiration breath-hold technique, and dashed lines indicate the 50% relative reduction. (A) heart V25, (B) mean LAD dose, and (C) left lung V25. The p-values are based on paired t-test. V25, relative structure volume receiving more than 25 Gy; LAD, left anterior descending coronary artery.
The dosimetric comparison also showed that the average NTCP for cardiac mortality was reduced from 2.9% on the FB plans to 0.7% on the DIBH plans and 1.8% on EIBH plans, representing relative reduction rate of 76% (p < 0.001) and 38% (p = 0.004), respectively. There were significant reductions in mean LAD dose and maximum LAD dose of DIBH plans in comparison with FB plans (Fig. 3B and 4B). EIBH plans resulted in a significant reduction of mean LAD dose (p = 0.005), but demonstrated no significant difference in maximum LAD dose (p = 0.647).
Using the inspiration breath-hold technique, the average left lung volume was increased from 881.6 (FB) to 1,575.3 cm3 (DIBH) and 1,106.0 cm3 (EIBH). The average irradiated lung volume was also increased from 155.1 (FB) to 253.5 cm3 (DIBH) and 197.8 cm3 (EIBH). Despite of the significant expansion of the left lung volume, there were no significant differences in left lung V25 among the three plans (Fig. 3C and 4C; p > 0.05).
Discussion and Conclusion
Since the late 1990s, various attempts have been tried to reduce the dose to the heart and lungs for the purpose of decreasing long term toxicity related to radiation therapy of breast cancer. Respiratory maneuvers, such as DIBH and EIBH technique, were proposed to reduce cardiac and lung dose. In these techniques, the heart is pushed down and out of radiation field by inflation of lungs (Fig. 5). Remouchamps et al. [9] reported initial clinical result of 3.6% reduction of heart V30 by moderate DIBH technique using ABC device. Using RPM system, Korreman et al. [10] evaluated various respiratory maneuvers and demonstrated the dosimetric benefits of free breathing gated breast cancer radiotherapy for the first time. In comparison with FB technique, not only DIBH but also EIBH technique demonstrated a significant reduction in V24 of heart, LAD, and lungs.
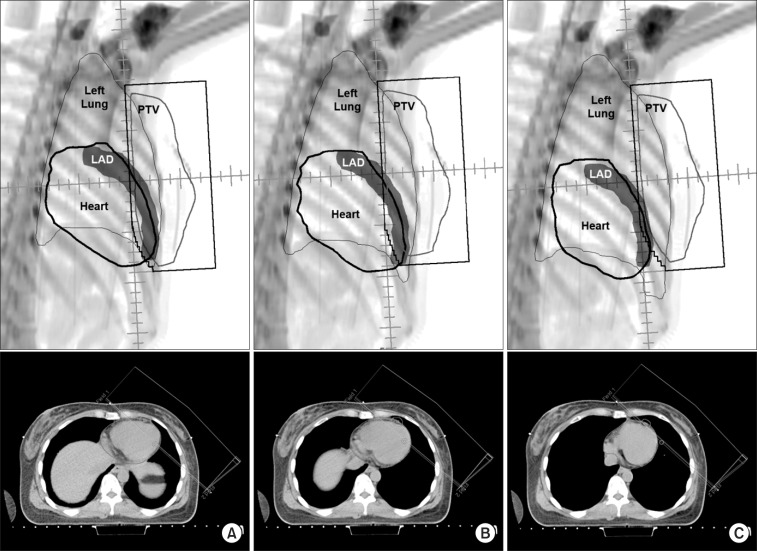
Beams eye views and axial computed tomography images from the medial tangential field for (A) free breathing, (B) end inspiration breath-hold, and (C) deep inspiration breath-hold plans. PTV, planning target volume; LAD, left anterior descending coronary artery.
As shown in Table 2, several authors have reported the advantage of respiratory maneuver in reduction of cardiac radiation dose for left-sided breast cancer [12,13,14]. There were some differences in reporting the results among these studies, e.g., depth of inspiration, target definition, prescription dose, and dosimetric parameters used in plan evaluation. Although it is not possible to provide a direct comparison, all of these studies demonstrated a significant reduction in cardiac dose using inspiration breath-hold technique. For DIBH technique, the relative structure volume receiving more than 24 to 30 Gy ranged from 0.6 to 2.45, representing 3.6% to 96% of relative reduction.
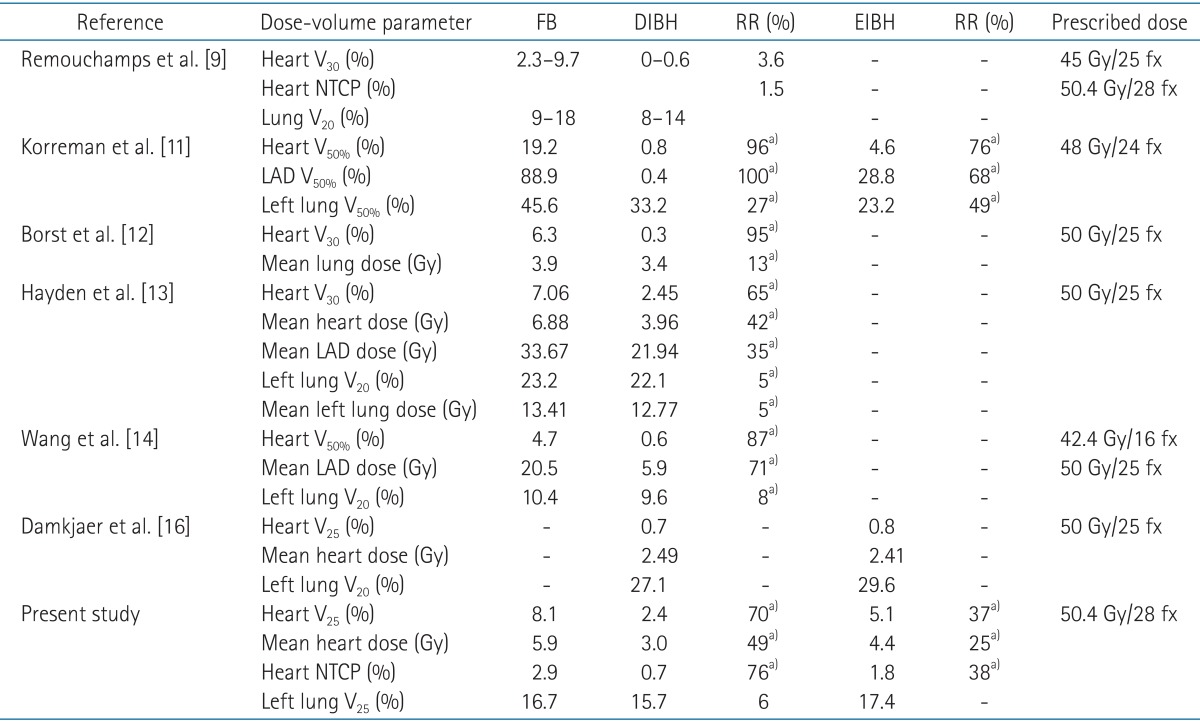
Dosimetric parameters for the plans using various respiration techniques demonstrated by previous studies
In the present study, DIBH plans demonstrated 70% relative reduction in heart V25 and 76% relative reduction in NTCP for cardiac mortality. Using EIBH technique, relative reductions in heart V25 and NTCP for cardiac mortality were 37% and 38%, respectively. Although there were several limitations in assessing the heart volume by the CT scan data, such as the different delineation scheme and uncertainty generated by beating movement of the heart, this study demonstrated substantial reduction of cardiac dose by inspiration breath-hold technique in coincident with former studies.
For the lung parameters, published data yielded conflicting results (Table 2). A significant reduction of dose to lungs using breath-hold technique was observed in the studies of Korreman et al. [10] and Borst et al. [12]. However, Hayden et al. [13] showed that there was no significant lung dose reduction in inspiration breath-hold technique compared to FB technique. In a pair of wedged tangential fields, irradiated lung volume can be increased using inspiration breath-hold technique, but this phenomenon is hampered by coincident increase of whole lung volume. As a result, there will be no significant changes among different breathing maneuvers in dose-volume parameters of lung. In our study, irradiated lung volume was increased by DIBH technique with the relative increase of 63%, and total lung volume also demonstrated 72% of relative increase. Consequently, our study demonstrated no significant reduction of lung dose by breath-hold technique.
The reproducibility of inspiration level should be considered in application of breath-hold technique for left-sided breast cancer patient. Various devices such as ABC device, RPM system, and Abches system were tried to monitor respiratory chest wall excursion, and to maintain inspiration level consistently. DIBH using ABC had been shown to produce immobilization of the lung and chest wall, with excellent intra- and inter-fraction reproducibility [9,14,21]. However, ABC apparatus requires additional resources, e.g., a digital spirometer and an air compressor in the treatment room. In addition, the compliance of the patients with a mouthpiece with viral filter, a nose clip and forced air pressure is of concern. A respiration-monitoring device, such as Abches system also used for DIBH treatment [17], but this system is not commonly installed in majority of institutions. Recently, Bartlett et al. [15] evaluated voluntary DIBH technique without any breathing control device. This randomized crossover study compared ABC assisted DIBH and voluntary DIBH in terms of setup reproducibility, normal tissue sparing and feasibility of delivery. The results demonstrate that the two techniques are similar in terms of these parameters. Another recent study showed that visually guided breath-hold method assisted by video goggles was more reproducible than audio-coached technique [16].
In the present study, we introduced easy to use and patient friendly in-house technique, named SRM system which is synchronized with RPM system. As in other recently reported DIBH techniques, our technique requires no additional patient-bothering equipment except for a LCD monitor and RPM system already installed in the treatment room. Using the SRM system from the training course, the visual feedback was easily applied to help the patients achieve an accurate, reproducible breath-hold level as quickly as possible, and maintain it comfortably during CT scan time. Furthermore, the accuracy of beam delivery in the correct inspiration phase or respiratory cycle is another merit of the RPM system. With SRM system, patients can see their animated graphical information of inspiration amplitudes with their time interval. As the RPM system gates the treatment beam, patients themselves can control beam delivery by regulating their own respiratory pattern during the treatment. If the patient releases the breath-hold or changes breathing amplitude, the RPM system can hold the beam immediately. Further study is ongoing to assess the benefit of the RPM system combined with SRM system for the real treatment in terms of positional reproducibility, treatment time, and patient compliance.
In the absence of clear threshold dose resulting in excess risk of radiation related toxicity, several authors have reported about the radiation related risks of heart disease and lung cancer after radiotherapy of left sided breast cancer. Henson et al. [5] pointed out the increased cardiac mortality ratio for left versus right tumor laterality, and the lung cancer mortality ratio for ipsilateral versus contralateral breast cancer, which were the consistent phenomenon during the third decade after exposure for women diagnosed with breast cancer during 1973-1982. No significant increased risk of cardiac mortality, however, was found for the patient receiving radiation therapy after 1983.
One of the main reasons why cardiac mortality ratio was not increased in patient irradiated after mid-1980s is considered to be the change of radiation therapy technique; omitting of internal mammary chain irradiation in breast cancer treatment. In the 1970s and early 1980s, most patients treated with tangential beam irradiation of the chest wall including internal mammary chain with estimated mean cardiac dose of around 14 Gy for left-sided and 9 Gy for right-sided tumor [22]. Since the 1980s, increasing number of patients received modern standard tangential irradiation for the whole breast after breast conserving surgery without internal mammary irradiation, resulting in estimated mean cardiac dose of 6 Gy and 2 Gy for left- and right-sided breast tumor, respectively. These results suggest that reduced cardiac dose in patient receiving radiation therapy since the 1980s resulted in reduced risk of radiation related cardiac mortality. Consequently, dose reduction to the heart and lungs is a necessity to decrease the risk of radiation-related mortality.
Even with modern radiation therapy techniques, some patients with unfavorable anatomy receive a substantially high radiation dose to the heart and lungs [23]. Moreover, in case of requiring internal mammary chain irradiation for selected patient with advanced breast cancer, more radiation dose is still given to the heart and lungs. In these cases, radiation techniques, e.g., DIBH would be required to reduce radiation dose to heart. There are some suggestions about the threshold value to select patients to whom such techniques would be beneficial. Using NTCP model-based estimation, Gagliardi et al. [24] predicted that probability of cardiac mortality 15 years after radiotherapy would be <1% if heart V25 was limited <10%. In the present study, 6 of 22 patients demonstrated heart V25 over 10% in FB plans (Table 3). All of these 6 patients achieved reduction in heart V25 to lower than 10% using DIBH and EIBH technique. Marks et al. [23] reported dramatic increases in the incidence of cardiac perfusion defects from 10%-20% to 50%-60%, if >5% of the left ventricle were included within the radiation field. Based on these results, Wang et al. [14] reported their experience of patient selection for DIBH treatment using two field tangential IMRT with the threshold heart volume of 10 cm3 within the radiation field (heart V50% >10 cm3). Until now, as far as the authors' knowledge, two different threshold values were suggested for 2 different end points. One is to limit <10% of heart volume in radiation field to reduce the risk of cardiac mortality under 1%, and the other is to prevent the cardiac perfusion defect, radiation field should encompass <2.5% of heart volume. According to the authors' interpretation by inference from heart V50%, previously reported studies using conventional tangential fields showed relatively large and wide range of irradiated heart volume from 4.7% to 19.2% of heart volume (Table 2). Therefore, the threshold value of <2.5% of heart volume seems to be too strict and the threshold value of <10% of heart volume would be more applicable in patient selection for inspiration breath-hold technique. Further research is required to reach a consensus about threshold dose to prevent long-term cardiac complication.
In conclusion, both DIBH and EIBH plans demonstrated a significant reduction of radiation dose to the heart in comparison with FB plan. In the training course prior to CT scan, SRM system was useful and effective in terms of positional reproducibility and patient compliance without any additional equipment such as ABC system and Abches device. Further research is required to determine the patient selection criteria for inspiration breath-hold technique.
Acknowledgments
The present research has been supported by the Gachon Research Foundation. The authors are grateful to the radiotherapists for their assistance in this study.
Notes
No potential conflict of interest relevant to this article was reported.