Retroperitoneal liposarcoma: the role of adjuvant radiation therapy and the prognostic factors
Article information
Abstract
Purpose
To evaluate the benefit of adjuvant radiation therapy (RT) for retroperitoneal liposarcoma (RPLS) following gross tumor removal.
Materials and Methods
We reviewed 77 patients with primary RPLS surgically treated between January 2000 and December 2013. Cases with gross residual disease were excluded. Tumor grade was evaluated according to the French Federation of Cancer Centers Sarcoma Group (FNCLCC) system. Adjuvant RT was delivered to 32 patients (42%) using external beam RT alone. Median follow-up time was 36 months (range, 5 to 169).
Results
Among 77 patients, 33 (43%) presented with well-differentiated, 31 (40%) with de-differentiated, 8 (10%) with myxoid/round and 4 (5%) with pleomorphic morphology. The RT group included less well-differentiated subtype than surgery group (28% vs. 53%). During follow up, 34 patients (44%) showed local recurrence. Local recurrence rate was lower in the RT group (38%) compared to the surgery group (49%). The 3-year local control rate (LC) was 55.6%, and the 3-year overall survival (OS) was 82.1%. Tumor histology and FNCLCC grade were significantly associated with local recurrence. There was no statistical significance of adding adjuvant RT in LC (p = 0.312). However, patients with tumor histology other than well-differentiated subtype showed marginally decreased local recurrence rate after adjuvant RT (3-year LC, RT 43.9% vs. no RT 35.3%; p = 0.087).
Conclusion
RPLS patients receiving RT experienced less local recurrence. We suggest that the addition of adjuvant RT may be related to improvement of LCs, especially in patients with non-favorable histologic subtypes.
Introduction
Soft tissue sarcomas are rare, heterogenous neoplasms, comprising for less than 1% of all newly diagnosed malignancies. Of all soft tissue sarcomas, only 10%–20% occur in the retroperitoneum [1]. The predominant histologic type of retroperitoneal sarcoma (RPS) is liposarcoma (47%), followed by leiomyosarcoma (29%) [2]. Classification of liposarcoma into subtypes, based on morphologic features and cytogenetic aberrations are widely accepted. The subtypes includes well-differentiated, de-differentiated, myxoid/round cell and pleomorphic, according to the World Health Organization (WHO) classification [3].
The prognosis of retroperitoneal liposarcoma (RPLS) is poor compared to other histologic types of RPS [4]. Surgical resection with negative resection margin is the only treatment modality providing chance of cure, while chemotherapy or radiation therapy (RT) without surgery is rarely beneficial, either used alone or with combination [5]. However, the large size, anatomical location and invasiveness of RPLS often prevents from achieving adequate margin. Also, RPLS tends to show a high rate of local recurrence even in cases with negative surgical margins [6]. Published retrospective data utilizing surgery alone have reported 5-yr local control rate (LC) mostly below 50% [7-9]. The high local recurrence rates for RPS supports a need for adjuvant RT as an effective local control modality.
Currently, there is no prospective randomized controlled trial confirming the potential benefit of RT for RPS, including RPLS, and the majority of published reports are single-institution, retrospective studies [10]. The aim of this study is to evaluate the benefit of adjuvant RT following surgery in RPLS patients. We also compared the efficacy of adjuvant RT according to tumor subtypes.
Materials and Methods
1. Patient population
We analyzed 77 patients with primary RPLS who underwent surgical resection between January 2000 and December 2013 at our institution. Patients with recurrent disease, previous RT history including preoperative RT, or gross residual disease after surgery were excluded from the study. We reviewed clinical data, surgical notes and pathologic results of the patients. Histologic type of tumor was reviewed and classified into 4 subtypes according to the WHO classification. Tumor grade was evaluated according to the French Federation of Cancer Centers Sarcoma Group (FNCLCC) system.
2. Treatment characteristics
The surgical aim of all patients was obtaining negative resection margins with surgical excision, and was defined as “R0 resection” if successfully achieved. If the resection margin was microscopically involved, it was defined as “R1 resection.” Otherwise, if the tumor was grossly removed but the surgical specimen was not available to assess margin, we described it as “RX resection.” Resection of contiguous organ (kidney, bowel, pancreas, and spleen) was done in 57 patients (74%) to achieve negative surgical margins.
Adjuvant RT was delivered to 32 out of 77 patients (42%) using external beam RT (EBRT) alone. The median total dose was 54 gray (Gy) (range, 44 to 60 Gy), and intensity-modulated RT (IMRT) was used in 12 patients (38%). Clinical target volume (CTV) was delineated with 3–5 cm margin from the tumor bed, with consideration of anatomical risky areas of recurrence. For some R1-resected patients, considering adjacent organ toxicities, a boost dose was delivered to high-risk CTV which included microscopically involved resection margin areas. Total dose and target volume margins were modified considering distance from dose-limiting structures including bowel, kidney and spinal cord. Regional lymph nodes were not irradiated in all patients.
Adjuvant chemotherapy was delivered to 6 patients (8%) after RT. All patients receiving chemotherapy showed dedifferentiated subtype and were classified as grade 2 or higher. The chemotherapy regimen consisted of doxorubicin 50 mg/m2, ifosphamide 8 g/m2, and mesna 2 g/m2 over 4 days for 3 cycles.
3. Statistical analysis
The estimates of LC, disease-free survival (DFS), and overall survival (OS) were calculated using the Kaplan-Meier method. Local control and survival outcomes were calculated from the date of surgery to the date of event or last follow-up. The chi-square test and bivariate correlation analysis were used to compare patient characteristics between patient groups. Multivariate analysis was performed using the Cox proportional hazards model. All tests were two-sided and considered statistically significant for p-value less than 0.05. We used IBM SPSS ver. 20.0 (IBM, Armonk, NY, USA) for the analysis.
Results
1. Patients characteristics
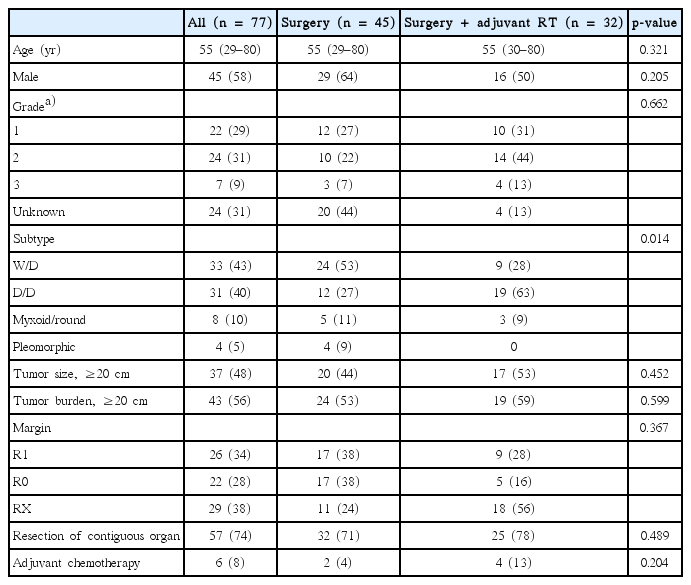
There were 45 male and 32 female patients. Median age at diagnosis was 55 years (range, 29 to 80 years). Among 77 patients, 33 (43%) presented with well-differentiated, 31 (40%) with de-differentiated, 8 (10%) with myxoid/round and 4 (5%) with pleomorphic morphology. The FNCLCC grade was evaluated in 53 patients; 22 (42%) were classified as grade 1, 24 (45%) were grade 2, and 7 (13%) were grade 3. Margin status was available in 48 patients, of which 26 (54%) were R1 resection. Tumor size was defined as maximum single tumor diameter, and tumor burden was calculated as sum of maximum tumor diameter for all respective tumors. Median tumor size was 19 cm (range, 5 to 60 cm) and median tumor burden was 21 cm (range, 10 to 86 cm).
A comparison of patient characteristics and pathologic status between groups treated with surgery only (surgery group) and with adjuvant RT following surgery (RT group) are summarized in Table 1. The surgery group included larger proportion of well-differentiated subtypes (24 patients, 53%) than the RT group (9 patients, 28%). Surgical margin status also differed between the two groups; the surgery group included more percentage of R0 resection patients (surgery group, 38% vs. RT group, 16%).
2. Clinical outcomes
During median follow-up time of 36 months (range, 5 to 169 months), 34 patients (44%) showed local recurrence and 19 (25%) died. Comparing between the two groups, 22 out of 45 patients (49%) experienced local recurrence in the surgery group while only 12 out of 32 patients (38%) showed local recurrence in the RT group. Patterns of failure in these groups are compared in Fig. 1. Of the 12 (38%) local failures in the RT group, 7 (22%) were within the RT field, 2 (6%) were marginal, and 3 (9%) were outside.
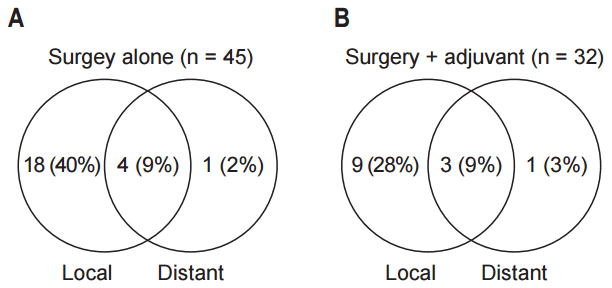
Patterns of failure in surgery alone group (A) and surgery followed by radiotherapy (RT) group (B) are depicted in diagram. Local, local recurrence; Distant, distant metastasis.
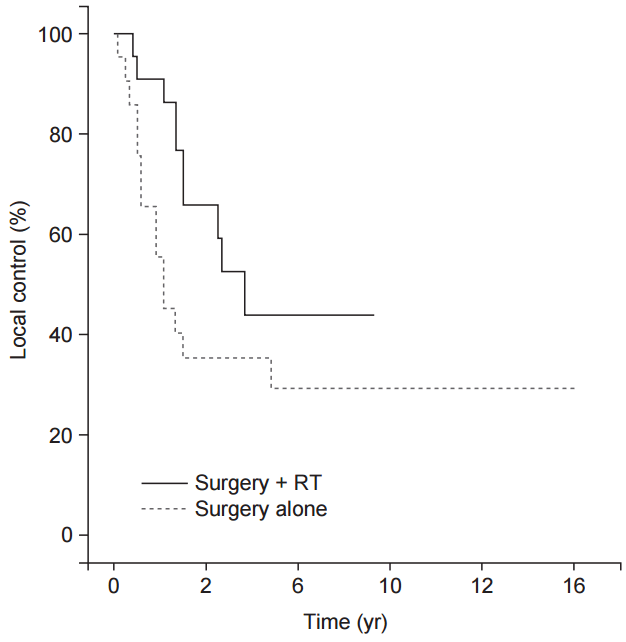
The 3-year LC, DFS and OS of all patients were 55.6%, 53.6%, and 82.1%, respectively. No significant differences were demonstrated between the two groups in 3-year LC (52.5% vs. 59.7%, p = 0.312), 3-year DFS (50.1% vs. 57.8%, p = 0.285) and 3-year OS (74.4% vs. 93.8%, p = 0.393) (Fig. 2).

The Kaplan-Meier survival curve determining local control rate (A), disease-free survival (B), and overall survival (C) of all patients are showed comparing surgery + radiotherapy (RT) group and surgery alone group.
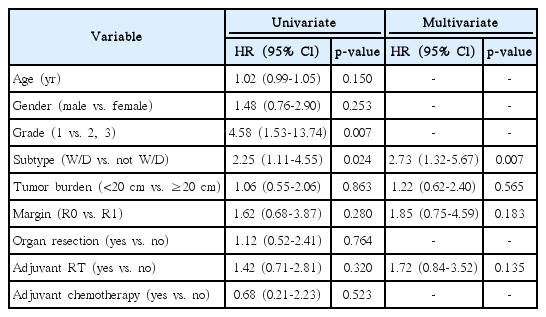
Univariate and multivariate analysis of prognostic factors determining local recurrence are shown in Table 2. By univariate analysis, favorable prognostic factors on LC included well-differentiated subtype (p = 0.024) and FNCLCC grade 1 (p = 0.007). We performed a multivariate analysis including subtype, tumor burden, surgical margin, and adjuvant RT, which are known as significant prognostic factors for LC in literature [11]. Tumor grade was excluded from the multivariate model because it was confounding with histologic subtype. On multivariate analysis, histologic subtype was statistically significant for LC (hazard ratio [HR], 2.73; 95% confidence interval [CI], 1.32 to 5.67; p = 0.007).
We performed a subgroup analysis according to histologic subtypes, for patients with well-differentiated subtype (n = 33) and patients with other subtypes (n = 43). For well-differentiated subgroup, there was no significant difference of 3-year LC (63.1% vs. 77.8%, p = 0.669) and 3-year OS (85.0% vs. 100.0%, p = 0.681) between the surgery group and the RT group. However, a subgroup analysis of other subtypes showed a marginal difference of 3-year LC between the two groups (35.3% vs. 43.9%, p = 0.087) (Fig. 3) while 3-year OS still had no difference (63.1% vs. 90.9%, p = 0.345). Including only dedifferentiated subtypes, 3-year LC also showed near-significant difference between the two groups (27.8% vs. 41.6%, p = 0.054).
Discussion and Conclusion
In this study, we reviewed primary RPLS cases and compared patients who were treated with surgery alone to patients treated with surgery plus postoperative RT. In our results, the RT group showed higher LC than the surgery group, even though the portion of well-differentiated subtype was significantly lower.
For surgically treated RPS patients, R0 resection is related with favorable prognosis [6,9,11]. However, the outcomes of surgery-only studies are not quite satisfying, with a 5-year LC ranging from 22% to 59% [9,12-14]. While there seems to be a pronounced necessity of adjuvant RT to achieve satisfactory local control, there is currently no level I evidence for RT in management of RPS, and results from retrospective data are also in debate. Thus, data from extremity soft tissue sarcomas have been generally extrapolated to the management of RPS [15].
Two randomized trials, one using intraoperative brachytherapy and the other using postoperative EBRT, support the use of RT in extremity or superficial trunk soft tissue sarcomas [16,17]. The latter trial was conducted by National Cancer Institute (NCI) to assess the impact of postoperative RT on local recurrence, OS, and quality of life after limb-sparing resection of extremity sarcomas. This study reported a significant decrease of local recurrence and superior quality of life within a median follow-up of 9.6 years, but there was no difference on OS [17].
Previous data from extremity tumors showed that disease-related deaths are almost always a consequence of distant metastasis, especially from lung metastasis. In contrast, for RPS patients, up to 75% of disease deaths are results of uncontrolled local diseases. This difference may reflect the tumor biology and the impact of anatomical location for each of the tumor sites [9,18]. This emphasizes that the role of adjuvant RT in RPS is no less important than that in extremity sarcomas. Nevertheless, there are practical difficulties in delivering effective postoperative RT for RPS; first, there is uncertainty of target volume delineation in the postoperative setting, and second, sufficient dose delivery is often limited due to the large size and adjacent location of primary tumor. Data from extremity sarcomas recommend to administer postoperative dose of 60 Gy in minimum, but the small bowel volume receiving dose greater than 45 Gy results in significant acute and late toxicities.
To date, no randomized trial have been completed comparing surgery with or without RT. The American College of Surgeons Oncology Group (ACOSOG) opened a phase III randomized trial (Z9031), comparing surgery alone versus preoperative RT followed by surgery. Unfortunately, this trial was terminated due to poor accrual. The European Organization for Research and Treatment of Cancer (EORTC) protocol 62092, a randomized phase III trial, randomized patients to en-bloc surgery with or without neoadjuvant RT (50.4 Gy in 28 fractions), and is currently enrolling patients.
Even though evidence are insufficient, postoperative RT is widely used for its advantages that it prevents delay of surgery, and that it is possible to deliver treatment selectively for patients with higher risk of recurrence, considering the margin status and pathology results. Thus, postoperative RT is mainly performed in high grade tumors and close or positive resection margins [19,20]. Sampath et al. [8] reported on 261 patients and showed that adjuvant RT was associated with significant improvement of local failure-free survival (LFFS) over surgery alone (5-year LFFS, 79% vs. 64%). Another large-volume retrospective study based on analysis of 165 patients by the French Cancer Center Federation Sarcoma Group also reported that adjuvant RT was related with better local control (5-yr LC, 55% vs. 23%) [21]. In a Surveillance, Epidemiology, and End Results (SEER) analysis of retroperitoneal or abdominal sarcoma patients between 1988–2005, RT provided survival benefit for patients with stage I disease by American Joint Committee on Cancer (AJCC) staging [22]. Recently, a case-control propensity score-matched analysis of the National Cancer Data Base was reported. The results showed that median survival was longer in postoperative RT group (89 months) than no RT group (64 months), and postoperative RT was significantly associated with improved overall survival compared to surgery alone (HR, 0.78; CI, 0.71 to 0.85; p < 0.001) [23].
Certainly, these results cannot be directly translated to evidence for benefit of adjuvant RT, because of the confounding factors including various RT indication of each institute and the diversity of patient characteristics. Nonetheless, these retrospective data including comprehensive analysis of numerous patients would support the role of RT, until the results of EORTC trial are reported afterwards.
According to our results, less patients recurred in the RT group (12/32, 38%) compared to the surgery group (22/45, 49%). Different distribution of histologic subtypes in the two groups should be highlighted on this comparison, since the proportion of well-differentiated subtype was significantly higher in the surgery group, which is a prognostic factor related to lower local recurrence. Moreover, a subgroup analysis excluding well-differentiated subtype suggested a potential benefit for local control of adjuvant RT against surgery only.
Histologic differentiation is one of the most important determinant of clinical outcome in liposarcoma patients. Well-differentiated RPLS mostly present as local recurrence but seldom metastasize, with a 5-year OS of 90%. Most of the treatment-related death results from local effects on adjacent organs. De-differentiated and myxoid/round cell subtypes each show 5-year OS of 75% and 60%–90%, respectively, also sharing the trait of low metastatic rates. Although dedifferentiated subtypes have potential of metastasis, the frequency is only about 10%–15%. Pleomorphic RPLS is the least common subtype but has high metastatic potential, showing a relatively poor 5-year OS of 30%–50% [24,25]. In our study, de-differentiated subtype was the second common subtype (40%) following well-differentiated subtype (43%), and excluding these two subtypes left only 13 patients. Since de-differentiated subtype has poor outcome compared to well-differentiated but still preferentially recurs in a local pattern, we can infer that de-differentiated RPLS might be the subtype that most benefits from adjuvant RT.
Our study have some limitations. First, the analysis was based on small-sample, retrospective data and thus exposed to bias. Second, margin status was not available in a rather large portion of patients (38%) compared to previous studies, which indicates even more biases and limitations toward accurately accessing the prognostic and predictive factors.
In conclusion, RPLS is a rare disease with little evidence of optimal treatment. As local recurrences are the primary cause of mortality, more aggressive modalities of local treatment strategy are required. Our study suggests that adding postoperative RT may be related to improved outcomes in RPLS, especially for subtypes other than well-differentiated.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.