Radiation for persistent or recurrent epithelial ovarian cancer: a need for reassessment
Article information
Abstract
Purpose
The role of radiotherapy (RT) was largely deserted after the introduction of platinum-based chemotherapy, but still survival rates are disappointingly low. This study focuses on assessing the clinical efficacy of RT in relation to chemotherapy resistance.
Materials and Methods
From October 2002 to January 2015, 44 patients were diagnosed with epithelial ovarian cancer (EOC) and treated with palliative RT for persistent or recurrent EOC. All patients received initial treatment with optimal debulking surgery and adjuvant platinum-based chemotherapy. The biologically effective dose (BED) was calculated with α/β set at 10. Ninety-four sites were treated with RT with a median BED of 50.7 Gy (range 28.0 to 79.2 Gy). The primary end-point was the in-field local control (LC) interval, defined as the time interval from the date RT was completed to the date any progressive or newly recurring disease within the RT field was detected on radiographic imaging.
Results
The median follow-up duration was 52.3 months (range 7.7 to 179.0 months). The 1-year and 2-year in-field LC rates were 66.0% and 55.0%, respectively. Comparisons of percent change of in-field tumor response showed similar distribution of responses among chemoresistant and chemosensitive tumors. On multivariate analysis of predictive factors for in-field LC analyzed by sites treated, BED ≥ 50 Gy (hazard ratio, 0.4; confidence interval, 0.2–0.9; p = 0.025) showed better outcomes.
Conclusion
Regardless of resistance to platinum-based chemotherapy, RT can be a feasible treatment modality for patients with persistent of recurrent EOC. The specific role of RT using updated approaches needs to be reassessed.
Introduction
Despite the significant therapeutic advances that have been achieved within the past decades, epithelial ovarian cancer (EOC) remains a lethal malignancy that is most often diagnosed at an advanced stage with bulky presentation. Relapse rates after primary therapy range from 40% up to as high as 85% [1-3]. Though whole abdominal irradiation (WAI) had historically been a widely-utilized treatment [3-7], the role of radiotherapy (RT) was largely deserted after the introduction of platinum-based chemotherapy that demonstrated therapeutic improvements superior to that attained by prior alkylating agents [1,2]. Nonetheless, patients continue to demonstrate disappointingly low survival rates and commonly require multiple-line chemotherapy due to eventual disease progression [8-10].
Depending on the extent of the debulking surgery and control of any residual disease, potential improvement in survival outcomes have been noted for not only earlier stages, but for stage IV patients as well. Though the most up-to-date National Comprehensive Cancer Network (NCCN) guideline revised in 2017 suggests “completion surgery as indicated by tumor response and potential resectability in selected patients” for stage II, III, and IV EOC [11], surgical approach is often anatomically challenging due to the characteristics of the pelvic cavity and R0 resection is often difficult to achieve without consequent morbidity [12-14]. As platinum-based chemotherapy became increasingly predominant, referrals for salvage or palliative RT of either unresectable EOC or EOC with solitary metastasis has also diminished due to assumption of potential cross-resistance. Though salvage treatment had been a challenge with outmoded RT techniques, contemporary advances such as intensity-modulated RT or stereotactic ablative RT has allowed radiation doses to be escalated with relatively lower rates of toxicity [15]. Thus this study focuses on assessing the clinical efficacy of RT in terms of its relation to chemotherapy resistance and the possibility of reviving its role for persistent or recurrent EOC.
Materials and Methods
1. Patients
From October 2002 to January 2015, 47 patients with persistent or recurrent EOC were referred for palliative RT at Seoul National University Hospital and Seoul Metropolitan Government Seoul National University Boramae Medical Center. Medical records were retrospectively reviewed as approved by the Institutional Review Board (No. J-1608-017-781 at Seoul National University Hospital and No. 20160704/26-2016-82/072 at SMG-SNU Boramae Medical Center) at both institutions.
All patients received initial treatment with optimal debulking surgery followed by adjuvant chemotherapy based on a taxane and platinum combination of up to a median of 6 cycles (range, 2 to 9 cycles). Initial tumor staging was done according to the International Federation of Gynecology and Obstetrics (FIGO) classification [16]. Persistent or recurrent EOC was defined at the time of referral for RT as any disease persisting or newly recurring after completion of initial treatment.
Patient history and physical examination was done at the time of referral for RT and additional work-up with imaging was done using computed tomography (CT), magnetic resonance imaging (MRI), or 18-fluoro-deoxyglucose positron emission tomography (FDG-PET). CA-125 levels before and after surgery, chemotherapy, and palliative RT were also collected. All records pertaining to therapy for EOC, including initial treatment prior to RT, and details on the technique, field, dose, and response after RT were examined. Two patients that had been diagnosed with simultaneous double primary cancer and 1 patient that did not receive adjuvant chemotherapy were excluded from the study. A total of 44 patients that received palliative RT were included in the final analysis. The median follow-up duration was 52.3 months (range, 7.7 to 179.0 months).
2. Pattern of palliative radiotherapy
The use of RT for palliative treatment of persistent or recurrent EOC was decided upon by relevant physicians. RT fields and dose were planned according to the site of relapse. For patients with multiple-site disease progression, the sites to be included in the irradiation field were decided upon at the discretion of the radiation oncologist based on the patients’ symptoms and relative tumor burden. Because dose and fractionation schemes varied considerably for each treatment site, the biologically effective dose (BED) was calculated using the following equation with α/β set at 10:
3. Evaluation of response
Response to chemotherapy and RT was evaluated based on review of radiologic imaging and categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 guideline [17,18]. In-field progression after RT was defined as detection of any progressive or newly recurring disease within the irradiated field. Tumor response to RT was compared as percent change from baseline or nadir. The best in-field tumor response was defined as the achievement of CR or PR at any point in time before the onset of in-field disease progression was detected. The percent change of tumor diameter was compared with baseline measurements obtained at the start of RT. The final infield tumor response was assessed based on the status of the in-field tumor control at the time of last follow-up. The tumor diameter was compared with the nadir measurement obtained during the time of follow-up after RT. If the measurement at baseline was the smallest, this measurement was used to assess the percent change of the final in-field tumor response.
4. Statistical analyses
The independent samples t-test and chi-squared test were used for univariate descriptive analysis. Progression-free survival (PFS) rates were estimated and compared using the Kaplan-Meier method and log-rank test. The primary end-point was the in-field local control (LC) interval, defined as the time interval from the date RT was completed to the date any progressive or newly recurring disease within the RT field was detected on radiographic imaging. PFS was defined as the time from the base of follow-up to the detection of first progression after initial surgery and chemotherapy. The Cox proportional hazards model was used for univariate and multivariate analyses to assess clinicopathological factors and hazards ratios (HR) with 95% confidence intervals (CI). All statistical analyses were performed in IBM SPSS Statistics ver. 22.0 (IBM, Armonk, NY, USA) and R version 3.2.3 available from the Comprehensive R Archive Network (CRAN) at http://www.r-project.org. A p-value of less than 0.05 was considered to be statistically significant.
Results
1. Patient characteristics
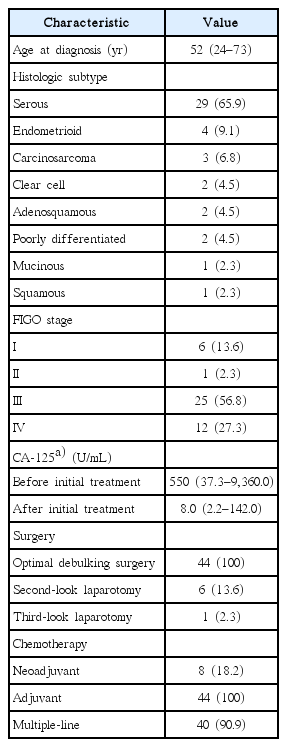
The majority of patients were initially diagnosed with serous EOC at an advanced stage. Prior to referral for RT, a large proportion of patients (90.9%) received multiple lines of chemotherapy due to either poor response to initial adjuvant chemotherapy or subsequent disease progression. Details on clinicopathologic characteristics and treatment at the time of initial diagnosis are presented in Table 1.
Table 2 summarizes patient characteristics at the time of referral for initial RT. Seventeen patients (38.6%) demonstrated resistance to prior adjuvant chemotherapy. Disease was limited to a single site in 12 patients (27.3%), whereas disease was simultaneously detected at multiple sites in 32 patients (72.7%).
2. Pattern of radiotherapy
Table 3 shows details of the patterns of RT by patient and site. Twenty-nine patients (65.9%) received a single course of RT throughout their full duration of therapeutic care and 15 patients (34.1%) received multiple courses. Five patients (11.4%) received re-irradiation to previously treated sites, which were all limited to the brain—3 patients received stereotactic radiosurgery (SRS) after initial whole brain RT (WBRT), 1 patient received a repeat WBRT, and 1 patient received a repeat SRS.
A total of 94 individual sites were treated with RT in the 44 patients, counting each site separately for patients that received multiple courses. For RT of metastatic lymph nodes (LN), subclavicular and para-aortic sites were the most common, each consisting of 11 of 35 cases. RT was given for bone metastases at 13 sites (13.8%), of which 6 sites were of the pelvic bones. RT target volumes in the pelvic cavity included soft tissue masses occurring in the pelvic wall or vaginal stump.
Excluding 34 brain metastases cases that were planned with either conventional RT or SRS and 2 bone metastases cases that were planned with conventional RT, 58 sites (61.7%) were treated with 3-dimensional conformal RT (3D CRT). The median BED was 50.7 Gy (range, 28.0 to 79.2 Gy). LNs were given higher BED at a median of 64.8 Gy (range, 40.8 to 79.2 Gy). BED ≥ 50 Gy was prescribed significantly more in patients being treated with palliative RT for LN metastasis (p < 0.001).
3. Outcomes of initial treatment
Of the 8 patients that received neoadjuvant chemotherapy, 5 patients had PR, and 3 patients had SD subsequent to optimal debulking surgery. After the completion of adjuvant chemotherapy, CR and PR was observed in 23 patients (52.3%) and 4 patients (9.1%), respectively. Of the 17 patients (38.6%) that showed resistance to adjuvant platinum-based systemic therapy, 3 patients demonstrated disease progression before all cycles of adjuvant cytotoxic therapy was completed. The overall median PFS after initial treatment was 16.2 months (range, 1.0 to 99.2 months). PFS was ≥12 months in 31 patients (70.5%) and ≥24 months in 12 patients (27.3%).
4. Outcomes of palliative radiotherapy
The median in-field LC interval after RT was 11.2 months (range, 1.4 to 166.3 months). In 18 patients (40.9%), the LC interval was ≥12 months. The median CA-125 level at the time of initial referral for RT was 49.5 U/mL (range, 2.2 to 1,650.0 U/mL) and at follow-up after completion of RT, the median was 23.2 U/mL (range 1.7 to 3,940.0 U/mL) and within normal limits.
Fig. 1 depicts waterfall plots for a comparison of in-field tumor response to RT based on the response to adjuvant chemotherapy and BED dose. Fig. 1(A) and 1(B) compares the percent change of tumor size achieved by each patient and Fig. 1(C) and 1(D) compares the percent change of tumor size between each treated site. Although chemoresistant cases showed more disease progression at final follow-up, waterfall plots demonstrate that these patients did not show poor tumor response to RT from the beginning. In both analyses, the best response to RT was similarly distributed among chemoresistant and chemosensitive groups and cases with the greatest tumor response had BED prescription of greater than or equal to 50 Gy. Table 4 describes best and final patterns of percentage change of tumors according to the site treated and dose prescribed.

Waterfall plots of in-field tumor response after radiotherapy according to the RECIST (Response Evaluation Criteria in Solid Tumors) criteria comparing (A) best response by patient, (B) final response by patient, (C) best response by site, and (D) final response by site.
Though assessment of statistical values was not possible due to the diversity of treatment regimens and follow-up schedules, it may be carefully suggested that there is no cross-resistance between chemotherapy and RT. However, it must also be noted that patients receiving 50 Gy or higher were predominantly treated for LN metastasis.
Table 5 summarizes results of univariate and multivariate analyses of predictive factors for in-field LC. Histologic subtypes were compared as two groups: (1) serous, others, and (2) clear cell, mucinous, endometrioid. Comparisons were made for all patients and for all cumulative number of treated sites. BED was compared with a cut-off value of 50 Gy based on the median prescribed total dose. BED greater than or equal to 50 Gy was a significant factor in univariate patient analysis, but did not retain its significance on multivariate analysis. RT given to regional pelvic cavity or nodal lesions demonstrated better LC intervals than that of lesions at distant sites. The site treated was the only significant factor on multivariate analysis by patient with an HR of 3.3 (CI, 1.6–9.5; p = 0.026) for distant lesions. On by site analyses, clear cell, mucinous, and endometrioid subtypes demonstrated significantly worse infield LC intervals on multivariate analysis (HR, 3.4; CI, 1.4–8.1; p = 0.001). Chemoresistant tumors (HR, 2.4; CI, 1.2–5.0; p = 0.019) had worse outcomes and tumors treated with BED ≥ 50 Gy (HR, 0.4; CI, 0.2–0.9; p = 0.025) demonstrated relatively better outcomes.
5. Outcomes of repeated palliative radiotherapy
Of the 15 patients that received additional courses of RT, the overall response to radiation was 66.7%: 2 patients had PD, 2 patients had SD, and progression was not assessable for 1 patient. Re-irradiation to a previously treated site was done for 4 patients. All 4 patients had received WBRT, where 3 patients were re-treated with SRS and 1 patient with repeated WBRT at a reduced dose from a previous 30 Gy to 24 Gy. The former group of patients showed PR in 1 patient and SD in the other 2. The latter patient that had received repeated WBRT had PR on follow-up. Of the 44 patients in the entire cohort, 1 patient had received 3 separate courses of RT. Initial irradiation was with SRS which resulted in near CR, but progressed 7.3 months later. A repeated SRS was given to the same lesion, but again showed disease progression 3.7 months afterwards. This patient had also received palliative RT to a LN in the lower neck which demonstrated a PFS of only 1.7 months.
Discussion and Conclusion
The drop of referral rates for palliative RT in patients with persistent or recurrent EOC were influenced by concerns that radiation may hinder maximal response and have cross-resistance to cytotoxic agents [19-21]. However, repeated use of cytotoxic agents often induces resistance and intolerable toxicity that worsens with time until ultimately the continuation of chemotherapy becomes difficult [20,21].
Several precedent documentations on palliative RT after chemotherapy failure have reported benefits regardless of chemoresistance, thus suggesting that resistance to chemotherapy does not indicate resistance to radiation [8,22,23]. In a report of 33 cisplatin-refractory patients that received palliative RT for symptomatic progressive disease, 93.9% experienced symptom remission, including 23 and 8 patients with symptomatic CR and PR, respectively [19]. Analysis of our data also demonstrated that patients with resistance to chemotherapy are able to achieve radiation response rates comparable to that of chemosensitive patients at some point in time before disease progression. Though chemoresistant patients showed a higher rate of eventual disease progression, our results suggest that resistance to chemotherapy does not correlate with poor response to radiation.
Another factor that also prompted the pullback of RT was that EOC was thought to be resistant to radiation regardless of the type of histology. Therapeutic plans lacked individualization and was generally approached as in high grade serous EOC, the most common subtype typically diagnosed at an advanced stage with bulky disease and formerly noted to have high rates of relapse after irradiation [6,24]. In the era where platinum-based chemotherapy prevailed, however, a subset of rare histologic subtypes including clear cell, endometrioid, and mucinous EOC that tend to present at earlier stages demonstrated to be relatively more resistant to chemotherapy [2,7,25]. The efficacy of RT in this subset was analyzed by Patel et al. [2] using the Surveillance, Epidemiology, and End Results program data from 2004 to 2011 and demonstrated a significant benefit of overall survival (54% vs. 44%) particularly for stage III patients. Though our series showed that overall response to RT in these rare subtypes was lower than that of serous types, it is difficult to make any inferences due to the low number of cases. In a study of 703 patients comparing several histologic subtypes, clear cell, endometrioid, and mucinous EOC were identified as a separate cluster demonstrating a statistically significant 40% reduction of disease-specific mortality with chemoradiotherapy in stages I and II [26].
Treatment of EOC in a curative setting demonstrated to require high doses of radiation. However, most patients diagnosed with EOC often present with disseminated disease involving the abdominal cavity and prescription of adequately high doses of radiation for the eradication of any bulky disease was difficult without inducing high rates of toxicity [20,27]. The current NCCN guideline for ovarian cancer recommends RT only in the palliative setting, where most references are from the early 2000s and data based on the use of modern-day RT techniques is difficult to find [11]. Because the role of RT has been nearly nonexistent after the growth of platinum-based chemotherapy, majority of data on historical WAI are based on 2D techniques of RT. According to the few studies that has been published in more recent years, the response rate after RT has been noted to be relatively high. In a study by Tinger et al. [1] on palliative RT for symptomatic lesions in 80 patients diagnosed with EOC, the overall response rate was 73%. Lee et al. [28] also reported a similar response rate of 65% in an analysis of 38 patients treated with salvage or palliative RT. A number of studies have reported the possible benefit of localized involved-field RT (IFRT) for locoregionally recurrent EOC. With the advances of modern RT techniques, unnecessary irradiation of normal tissues can be avoided and has allowed for dose escalation with relatively lower rates of toxicity [15,29]. In a study of 102 EOC patients treated with definitive IFRT at doses greater than or equal to 45 Gy, Brown et al. [30] demonstrated a 5-year in-field disease control rate of 71%. In 35%, patients had no evidence of disease after more than 2 years of follow-up. In another study by Albuquerque et al. [31], retrospective analysis of 27 patients that received IFRT with a median dose of 50 Gy in 25 fractions demonstrated locoregional failure-free survival rates of 70% at 5 years. Though the majority of cases in our series received low doses set at palliative aim, results of BED comparisons showed that patients receiving 50 Gy or higher prescriptions had better clinical outcomes for in-field LC.
Though our study is a review of an experience greater than 10 years, limitations are unavoidable due to the small number of available patients and heterogeneity of treatment patterns. The extent of disease progression and type of systemic therapy being administered was diverse for each patient at the time of referral for RT. Follow-up after palliative RT were mainly assessed and planned by relevant gynecology or hematology oncologists, thus radiologic imaging of the irradiated field were obtained at various intervals after completion of RT. Another limitation is the dose fractionation schedules that vary not only between sites, but individual patients as well. Though the BED was calculated for improved comparison, there were no standards for the total dose prescribed. BED ≥ 50 Gy was prescribed significantly more in patients receiving palliative RT for LN metastasis, thus our results are prone to possible selection bias and needs careful approach to interpretation.
In summary, the role of RT needs reassessment for patients with persistent or recurrent EOC regardless of initial resistance or gradual development of intolerance to platinum-based chemotherapy. Because the overall outcomes of EOC are poor and often demonstrate frequent relapse, earlier referral for RT is suggested such that salvage or palliative treatment can be integrated before increase of tumor burden. With the availability of modern-day techniques for more conformal planning and sparing of normal tissues, prescription of higher radiation doses may be possible.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.