Volumetric changes in the lumpectomy cavity during whole breast irradiation after breast conserving surgery
Article information
Abstract
Purpose
This study was performed to evaluate the change in the lumpectomy cavity volumes before and after whole breast radiation therapy (WBRT) and to identify factors associated with the change of volume.
Materials and Methods
From September 2009 to April 2010, the computed tomography (CT) simulation data from 70 patients obtained before and after WBRT was evaluated. The lumpectomy cavity volumes were contoured based on surgical clips, seroma, and postoperative changes. Significant differences in the data from pre-WBRT CT and post-WBRT CT were assessed. Multiple variables were examined for correlation with volume reduction in the lumpectomy cavity.
Results
The mean and median volume reduction in the lumpectomy cavity after WBRT were 17.6 cm3 and 16.1 cm3, respectively with the statistical significance (p < 0.001). The volume reduction in the lumpectomy cavity was inversely correlated with time from surgery to radiation therapy (R = 0.390). The presence of seroma was significantly associated with a volumetric change in the lumpectomy cavity after WBRT (p = 0.011).
Conclusion
The volume of lumpectomy cavity reduced significantly after WBRT. As the time from surgery to the start of WBRT increased, the volume reduction in the lumpectomy cavity during WBRT decreased. A strong correlation was observed between the presence of seroma and the reduced volume. To ensure appropriate coverage and to limit normal tissue exposure during boost irradiation in patients who has seroma at the time of starting WBRT, repeating CT simulation at boost planning is suggested.
Introduction
Breast conserving surgery followed by whole breast irradiation for early breast cancer is accepted as the standard treatment [1-3]. In Korea, 41.9% of all breast cancer patient received conserving surgery in 2004, and the rate of conserving surgery is gradually increasing [4,5].
Radiation therapy (RT) after breast conserving surgery is consisted of whole breast radiation therapy (WBRT) over 5 to 6 weeks plus a boost to the tumor bed. Numerous studies demonstrated that most local recurrences occur adjacent to the tumor bed or within the boost field [2,6,7], and boost irradiation has been shown in randomized studies to significantly reduce the risk of local recurrence [8-10].
Boost irradiation is delivered to the lumpectomy cavity with margins after 6 weeks of WBRT. Computed tomography (CT) simulation for the boost irradiation often uses the lumpectomy cavity identified on simulation CT scans acquired before the initiation of WBRT. Recent studies have reported the potential for the volume reduction in the lumpectomy cavity during WBRT [11-13]. Therefore, if single CT scan is used to plan the boost irradiation in a patient with a tumor bed that shrinks dramatically during the course of WBRT, the dosimetric coverage of tumor bed may be less optimal and excess normal tissues may receive unnecessary radiation [12,13]. The objectives of this study were to examine the changes of volume in the lumpectomy cavity after WBRT and to identify any factors that might predict a large volumetric change to select subgroup of patients who might benefit from repeat CT simulation for boost planning.
Materials and Methods
Between September 2009 and April 2010, 70 women undergoing breast conserving surgery and WBRT were included in this study. CT simulation of the breast were obtained in two sessions, the first CT was performed 5 days before the start of whole breast irradiation and the second CT was performed 2 days before the completion of 50.4 Gy WBRT.
Breast volumes were contoured on both CT scans according to the National Surgical Adjuvant Breast and Bowel Project B-39/RTOG 0413 protocol [14]. Contouring of the lumpectomy cavities on CT was guided by the presence of surgical clips, seroma, and other surgical changes. The CT planning and volumetric calculations were carried out using Phillips Pinnacle treatment planning system version 8.0 (Phillips Medical Systems, Andover, MA, USA).
The patient and tumor characteristics are listed in Table 1. Median age at initiation of RT was 47 years (range, 23 to 66 years). Body weights were in a range of 43.9 to 76.5 kg with a median weight of 58 kg. The most common pathology was infiltrating ductal carcinoma. Forty four patients were T1 tumors, 21 had T2 tumors, and 5 were preinvasive tumors (Tis). The most common tumor location was the upper outer quadrant. 15 patients (21.4%) with seromas were identified in CT scans before the start of WBRT. The time from surgery to start of WBRT was evaluated for correlation with volumetric change in the lumpectomy cavity and breast. 38 patients (54.3%) started WBRT within 2 months, 17 patients (24.3%) between 2 and 4 months, and 15 patients (21.4%) after 4 months from surgery. A total of 29 received a doxorubicin based chemotherapy (Table 1).
Significant differences in the data from pre-WBRT CT and post-WBRT CT were assessed using Student's paired t-test. Univariate analysis using Fisher's exact test was performed to determine the level of prognostic significance of selected factors in predicting the level of change from pre-WBRT CT and post-WBRT. For all statistical tests, p < 0.05 was considered significant. SAS ver. 9.1 (SAS Institute Inc., Cary, NC, USA) was used for all analysis.
Results
The volume reduction in the lumpectomy cavity was noted in 83% (58/70). The mean volume of the lumpectomy cavity before and after WBRT were 50.4 cm3 (range, 9.8 to 146.8 cm3) and 40.7 cm3 (range, 7.7 to 143.9 cm3), respectively. The lumpectomy cavity volume decreased by a mean value of 17.6% (range, -73.5 to 48%) (p < 0.001) and median value of 16.1%. The mean breast volumes before and after WBRT were 416 cm3 and 415 cm3, respectively, representing a mean change of 0.3% (range, -3.3 to 9.3%) (p = 0.394) and median change of 0.6% (Table 2).
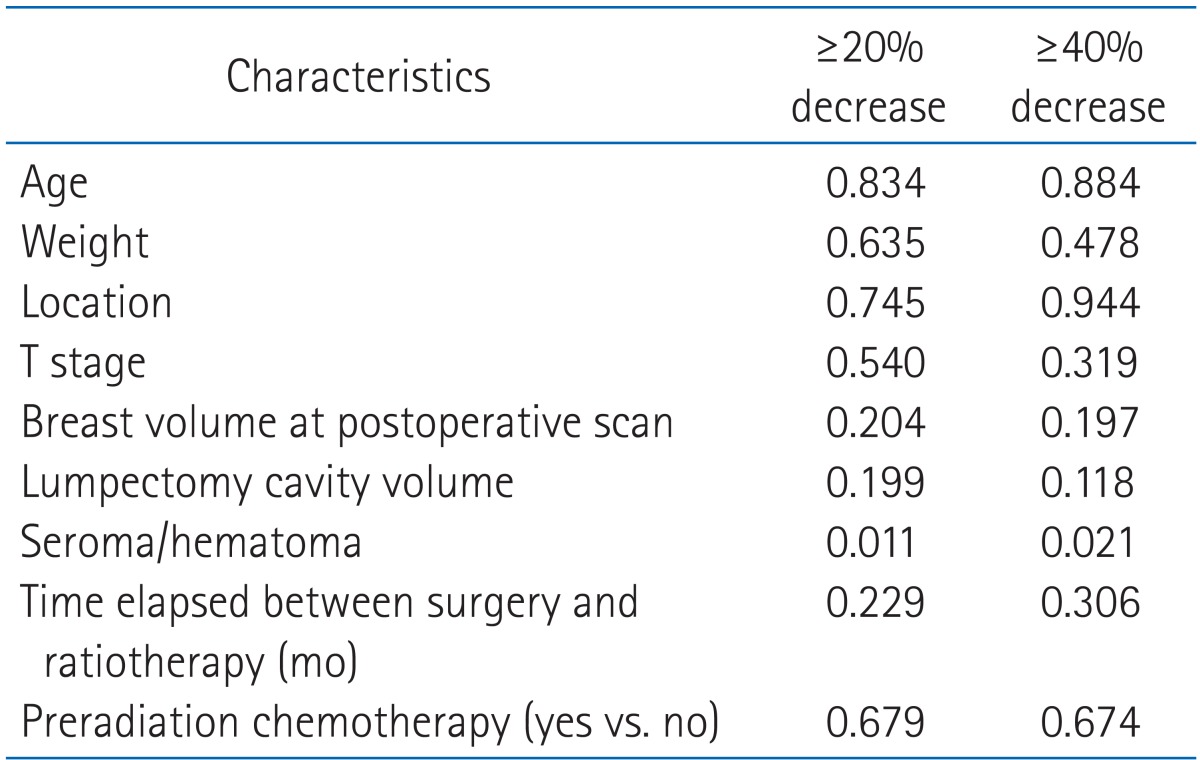
On univariate analysis, only the presence of seroma at the initiation of WBRT was significantly associated with a volume reduction in the lumpectomy cavity of 20% or greater and 40% or greater (p = 0.011, for ≥20%; p = 0.021, for ≥40%). Age, weight, location of tumor, T stage, breast volume, lumpectomy cavity volume, time to RT, and pre-radiation chemotherapy were not significantly associated with volume reduction of the lumpectomy cavity (Table 3).
The volume reduction in the lumpectomy cavity was inversely correlated with time elapsed from the lumpectomy to the start of radiation therapy (R = 0.390, p = 0.001) (Fig. 1). As the time from lumpectomy to start of radiation therapy increased, the extent of volume reduction in the lumpectomy cavity appears to be decreased but there was no statistical significance (p = 0.229, for ≥20%; p = 0.306, for ≥40%) (Table 3). However, if length of time from the lumpectomy to start of radiation therapy was subdivided into 3 groups (≤2 months, 2.1-4 months, ≥4 months), a statically significant difference in volume reduction in the lumpectomy cavity between 2 groups (≤2 months vs. >4 months) were found (p = 0.010). A volume reduction in the lumpectomy cavity was not significantly different when patients pre-treated with chemotherapy were compared with those who were not (p = 0.679, for ≥20%; p = 0.674, for ≥40%) (Table 3).
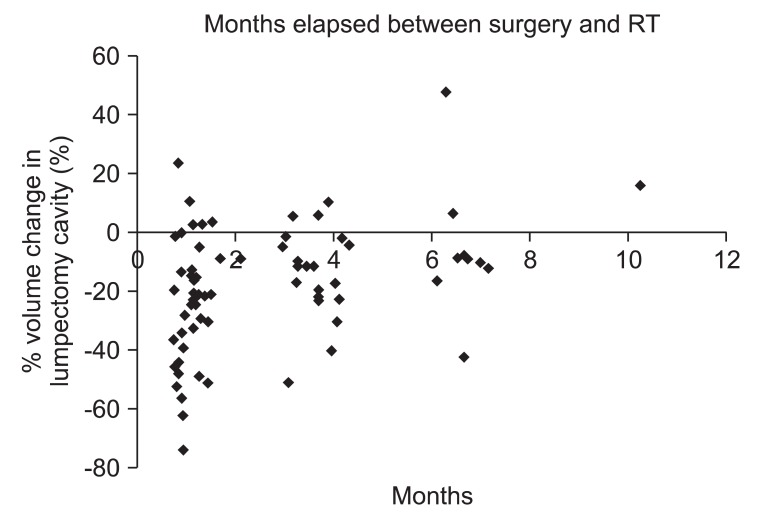
Scatter graph demonstrating a trend of the lumpectomy cavity shrinkage as time elapses from surgery. Each point represents a single lumpectomy cavity. The relative reduction in lumpectomy cavity volume demonstrates an inversely propotional trend when compared with time elapsed since surgery (R = 0.390). RT, radiation therapy.
Discussion and Conclusion
For the maximum local tumor control, boost irradiation field should accurately encompass the lumpectomy cavity [8-10], although the technique of delivering the boost is not standardized. From 10 to 67% of scar based boost plans were inaccurate in defining the boost volume when compared with surgical clip based plans [15-17]. And 70 to 80% of scar based plans resulted in geographical misses or modifications when compared with CT based plans [18-20]. CT based planning has become more popular in practice over the last several years and could reduce the geographical misses [21].
Boost irradiation is delivered immediately after WBRT. In many centers, single CT simulation obtained 5 to 7 weeks before the boost RT simulation is used to delineate the lumpectomy cavity. However, dynamic processes of complex tissue remodeling and healing both before and during the course of WBRT are occurring within the lumpectomy cavity. And these changes are account for significant reduction of the lumpectomy cavity volume, which may lead to the geographical misses or excessive normal tissue irradiation if single CT scans obtained before the course of WBRT are used for the boost planning [12,13,20-25].
Oh et al. [11] reported 22.5% of mean volume reduction in the lumpectomy cavity after WBRT. According to other investigators, a volume reduction were ranged from 25% to 64% [12,22,23,26]. In our study, volume reduction in the lumpectomy cavity was noted in 83% (58/70) patients, representing mean volume reduction in the lumpectomy cavity of 17.6% (range, -73.5 to 48%) (p < 0.001) and median of 16.1%. Hepel et al. [20] reported a mean volume reduction in the lumpectomy cavity during the course of WBRT of 52% and they attributed volume reduction to a decrease in postoperative seroma size. However, many other investigators [11,12,22,23,25,26] including our data demonstrated significant volume reduction in the lumpectomy cavity in patients who had no seroma during the course of WBRT, suggesting that dynamic remodeling processes of healing in the lumpectomy cavity also account for volume reduction.
Conversely, 15.7% (11/70) patients had an increase in lumpectomy cavity volume during WBRT, with a percentage increase of 1.1% to 17.9%. WBRT triggering inflammatory reaction may contribute to a swelling of tissue around lumpectomy cavity which is revealed as an increase in lumpectomy cavity volume.
The volume of breast was not significantly changed during WBRT in our analysis (p = 0.394) contrary to the significant volumetric change of the lumpectomy cavity. Other authors [11,12,20] also demonstrated minimal changes of breast volume during the course of WBRT.
Hepel et al. [20] reported that initial lumpectomy cavity volume of >15 cm3 correlated with a greater likelihood of a decrease in volume. Tersteeg et al. [22] reported a linear correlation between absolute volume of the lumpectomy cavity and the absolute volume reduction. A study by Flannery et al. [23] suggested a guideline of repeating CT simulation, which is the patients who has the large cavities (>30 cm3). Unlikely other investigators, Prendergast et al. [12] could not find significant statistical relationship between the volume of the lumpectomy cavity and the reduction. Our results also revealed no significant statistical relationship between the volume of cavity and the reduction (p = 0.199, for ≥20%; p = 0.118, for ≥40%). Oh et al. [11] reported that body weight and the time elapsed from surgery to RT initiation were inversely correlated with volumetric changes, a findings that was not a significant variables in our study. Other investigators [12,20] also reported no significant association between body weight and volumetric changes.
Prendergast et al. [12] reported that the rate of change was inversely proportional to the duration from surgery to radiation therapy initiation, however, no factors studied predicted large volumetric change in his study. Other authors [20-25] also consistently reported that time from surgery to radiation therapy initiation was inversely correlated with volume reduction of the lumpectomy cavity. In our study, a volume reduction in the lumpectomy cavity was inversely correlated with time elapsed from lumpectomy to start of radiation therapy (R = 0.390, p = 0.001) (Fig. 1). Although, the time from lumpectomy to start of radiation therapy was not significantly associated with the extent of volume reduction in our study, patients started radiation therapy within 2 months from surgery more likely to have a greater volume reduction (≤2 months vs. >4 months, p = 0.010). Weed et al. [25] reported similar relationship between the lumpectomy cavity volume and time elapsed from surgery but found that the volume reduction is minimal after 40 days postoperatively. Oh et al. [11] demonstrated continued volume reduction in the lumpectomy cavity past 150 days postoperatively. Our findings showed that the reduction in cavity volume is continued past 60 days postoperatively. Therefore, it may be reasonable to expect a greater volume reduction in the lumpectomy cavity after WBRT when the time from surgery to radiation therapy initiationis shorter. For these patients, repeating CT simulation for boost planning may be warranted.
The presence of seroma significantly induced extensive volumetric reduction during WBRT in our study and these changes could have an impact on the accuracy of boost irradiation planning (Fig. 2). Significant volume reduction in the lumpectomy cavity may also be of concern when boost irradiation is based on the initial CT simulation before WBRT.

Changes in the lumpectomy cavity before and after whole breast irradiation in the same geographical computed tomography slice at the same radiation treatment center. (A) Seroma has been decreased in volume. (B) Lumpectomy cavity appears to have been contracted with the movement of surgical clip.
Some institutions have been delivering the boost during WBRT by using a simultaneous integrated boost (SIB) technique which is irradiating differential dose to the tumor bed from day 1 of treatment [27-29]. However, Sharma et al. [13] reported that SIB technique still has the potential of leading to unnecessary normal tissue toxicity and dose inhomogeneity as the volume of lumpectomy cavity is reducing continuously during the WBRT.
Oh et al. [11] concluded that a separate boost CT simulation at the end of whole-breast irradiation is not needed for adequate coverage of the above-mentioned excision cavity boost despite of significant volume reduction in the lumpectomy cavity since the CT simulation obtained before the initiation of breast radiation adequately covers the excision cavity. However, large volume reduction of the lumpectomy cavity may lead to excessive normal breast tissue irradiation and also have an impact on the accuracy of boost irradiation planning, leading to significant effects on dose homogeneity in the treatment volume. Huh et al. [24] demonstrates that failure to consider patient's anatomic change during the period of radiation therapy could lead to complications associated with high-dose inhomogeneity. According to the study on the change of seroma volume during WBRT [13], mean and median reduction in seroma volume during radiation were 39.6% and 46.2%, respectively, which is the data suggesting the need for CT boost planning before boost irradiation to ensure appropriate coverage. Hepel et al. [20] and Flannery et al. [23] also suggested that repeating CT simulation at boost planning will allow for accurate delineation of the at-risk volume.
The end point of our study was evaluating physical change based on CT image and not local control or toxicities. And we presumed that the lumpectomy cavity is the best surrogate for the pathologic tumor bed. Prospective studies are warranted to quantify the effect of volume reduction in the lumpectomy cavity on dose distributions, acute and long-term toxicities, and local control.
In conclusion, the results of our study have shown that a significant volume reduction of the lumpectomy cavity after WBRT. As the time from surgery to the start of WBRT increased, the volume reduction in the lumpectomy cavity during WBRT decreased. A strong correlation was observed between the presence of seroma and the volume reduction. To ensure appropriate coverage and to limit normal tissue exposure during boost irradiation in patients who have seroma at the time of starting WBRT, repeating CT simulation at boost planning is suggested.
Acknowlegments
This work was supported by Grant from Inje University, 2009.
Notes
No potential conflict of interest relevant to this article was reported.