Postoperative radiotherapy for endometrial cancer
Article information
Abstract
Purpose
To investigate the prognostic factors and effectiveness of postoperative radiotherapy alone for endometrial carcinoma.
Materials and Methods
Sixty four patients with stage I-III endometrial cancer (EC) treated with postoperative radiotherapy alone between January 1989 and December 2008 at the Keimyung University Dongsan Medical Center were chosen for the present study. Typically, total hysterectomy, salpingo-oophorectomy and lymphadenectomy were performed on the patient's pelvis. Total dose from 50.4 Gy to 63 Gy was irradiated at pelvis or extended field. Thirteen patients were treated with Co-60 or Ir-192 intracavitary radiotherapy. Follow-up periods were from 7 to 270 months, with a median of 56 months.
Results
Five year overall survival (OS) rate was 58.7%, respectively. Five year disease-free survival (DFS) rate was 59.2%, respectively. In univariate analysis for OS and DFS, stage, menopausal age, type of operation, serosal invasion, and lymph node involvement were found to be statistically significant. Histologic type was marginally significant. In multivariate analysis for OS and DFS, stage, types of operation, histologic type were also found to be statistically significant. Treatment failure occurred in 14 patients. The main pattern of failure was found to be distant metastasis. Time to distant metastasis was from 3 to 86 months (median, 12 months). There were no grade 3 or 4 complications.
Conclusion
Stage, types of operation, and histologic type could be the predictive prognostic factors in patients. We contemplated postoperative radiation as effective and safe treatment method for EC. Additional treatment would be needed to reduce distant metastasis.
Introduction
According to annual report of cancer statistics in Korea in 2008, endometrial cancer (EC) is considered as the third common malignancy of the female genital tract in Korea and represents 2.1% of all the cancers. However there has been a gradual increase in the incidence of EC [1]. Maximal rate of incidence was observed in people aged between 50 and 70 years and postmenopausal women were the mostly affected. There were other risk factors including hormone replacement therapy (HRT), diabetes mellitus (DM), hypertension, etc., in association with EC. It has been reported that endometrial carcinoma has a relatively good outcome after multimodality treatment with a combination of surgery and adjuvant radiotherapy (ART) [2]. In the past, there have been certain large randomized trials in the world with respect to comparison of ART with surgery alone and it has been reported that ART possessed benefit for locoregional control, but had no benefit on overall survival [3-7]. There was no difference of distant metastasis between ART and surgery. Late complications of ART were more than surgery, but most of the complications were low-grade complications [3-7].
This study was designed to investigate retrospectively the prognostic factors for survival and effectiveness of postoperative radiotherapy alone in the patients with endometrial cancer in a single institute.
Materials and Methods
1. Patient characteristics
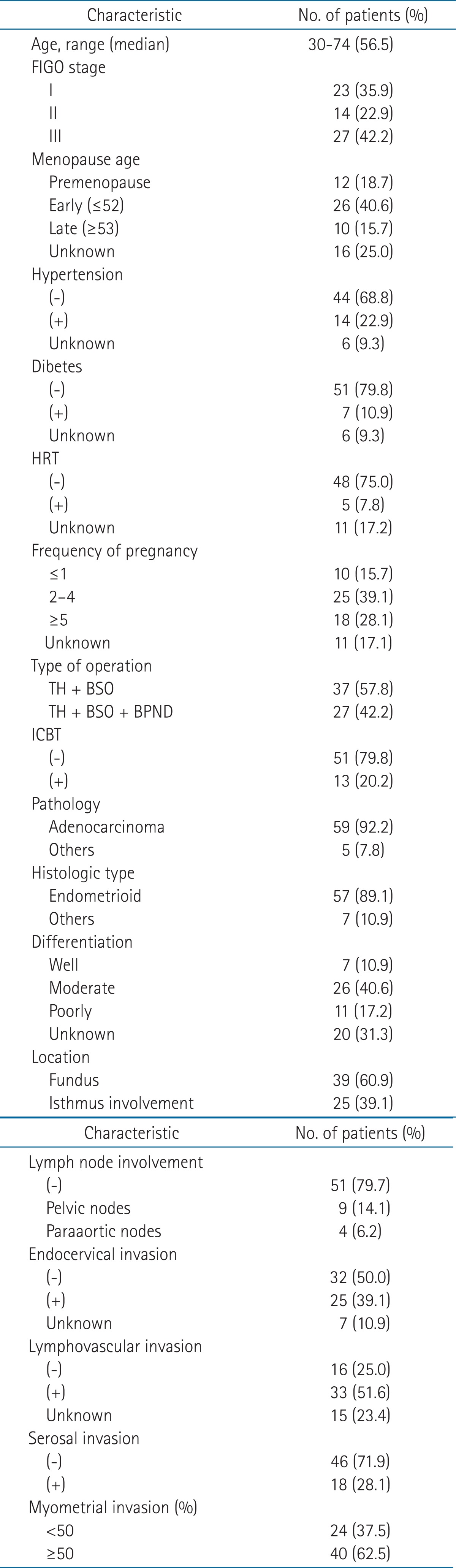
We retrospectively reviewed 139 patients with EC who were referred for radiotherapy at Keimyung University Dongsan Medical Center from January 1989 to December 2008. Of 139 patents, 88 patients had postoperative radiotherapy alone excluding patients treated with chemotherapy or hormone therapy. Sixteen did not have regular follow-up, 3 did not complete radiotherapy treatment, 2 did not had any confirmed pathologic diagnosis, 1 was in International Federation of Gynecology and Obstetrics (FIGO) 2010 stage IV, 1 had only Intracavitary brachytherapy and in 1 case diagnosis was changed after operation. Therefore, they excluded from this study. Therefore, we analyzed 64 patients with histologically-confirmed FIGO stage I-III. Median age of the patients was 56.5 years (range, 30 to 74 years). Of 64 patients, 23 patients were in stage I, 14 patients were in stage II, and 27 patients were in stage III EC. Moreover, we analyzed prognostic factors such as hypertension, diabetes, smoking, hormone replacement therapy, the number of pregnancy, menopausal age, myometrial invasion (MI), etc. These characteristics of patients are shown in Table 1.
2. Radiotherapy
Of 64 patients, 51 had external beam radiotherapy (EBRT) and 13 had EBRT and intracavitary brachytherapy (ICBT). The EBRT was performed on patient with lymphovascular invasion (LVI), endocervical invasion, parametrial invasion and pelvic lymph node (LN) metastasis. The EBRT was delivered basically in the prone position and 4-portal box technique, a belly board was used since 2005. The field of treatment was defined to include the upper two thirds of the vagina, paravaginal tissues, pelvic LNs and presacral LNs. The upper border was defined at the L5-S1 interspace. In patients with common iliac or paraaortic LN involvement, upper border was extended up to T11-12 interspace. The inferior border was defined at the lower margin of obturator foramen. The lateral border was defined at 2 cm margin from the lateral border of bony pelvis. The anterior border was anterior aspect of the pubic symphysis. The posterior border was defined at S2-S3 interspace. A total dose of 50.4-63 Gy (median, 54 Gy) was delivered, with a daily dose of 1.8 Gy. Midline shielding was added in 48 patients after 36-45 Gy (median, 45 Gy). The ICBT was performed subsequent to EBRT. Indications of ICBT were positive surgical margin or endocervical invasion. The ICBT was delivered with 192Ir high dose rate after-loading using vaginal mould. Total dose of 25-40 Gy (median, 30 Gy) was delivered, with 5 Gy at the vaginal surface or 0.5 cm from vaginal surface once a day.
3. Follow-up
Median follow-up period was 56 months (range, 7 to 270 months). During the entire period of radiotherapy (RT), physical examinations and complete blood count were performed once a week. After the completion of RT, patients were evaluated for disease status, recurrence and distant metastasis every 3 months until 2 years and every 6 months after 2 years. Overall survival (OS) was calculated from the date of operation to the time of death or last follow-up date. Disease-free survival (DFS) rate was calculated from the end of RT to the time of recurrence or distant metastasis. Complication was evaluated using the grading criteria of the Radiation Therapy Oncology Group (RTOG).
4. Statistical analyses
The Kaplan-Meier method was used to estimate OS rates and DFS rates. Univariate analysis for survival rate used log-rank test to evaluate the association between survival time and various factors. Multivariate analysis of prognostic factors for survival rate used Cox proportional hazards regression to evaluate the association between survival time and various factors. In addition, p < 0.05 was considered as statistically significant. The statistical analyses were performed with PASW ver. 18.0 (SPSS, Chicago, IL, USA).
Results
1. Survival
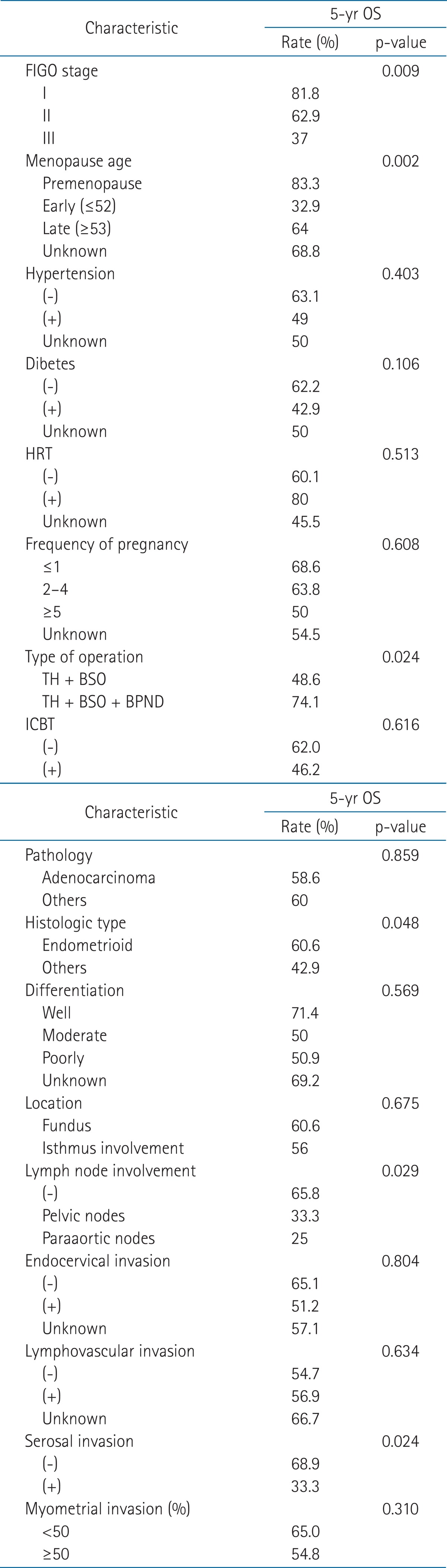
The median OS was 56 months (range, 7 to 270 months). Two year and 5-year OS rates were 68.8% and 58.7%, respectively. The median DFS was 53 months (range, 1 to 268 months). Two-year and 5-year DFS rates were 62.5% and 59.2%, respectively (Fig. 1). The 5-year OS rates of stage I, II, and III were 81.8%, 62.9%, and 37%, respectively. The 5-year DFS rates of stage I, II, and III were 82.6%, 63.5%, and 37%, respectively. Of 64 patients, 33 had no evidence of disease and one patient survived with disease and 30 patients expired. Thirteen patients expired due to disease progression and 17 expired due to other causes (Fig. 2).
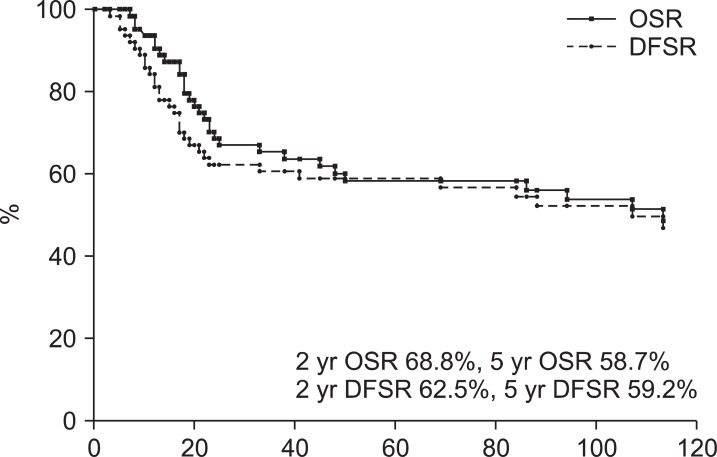
Survival rates in patients treated postoperative radiotherapy for endometrial cancer. OSR, overall survival rate; DFSR, disease-free survival rate.
2. Prognostic factors
Various other factors such as stage, menopausal age, hypertension, diabetes mellitus, hormone replacement therapy, frequency of pregnancy, type of operation, pathology, histologic type, differentiation, location, LN involvement, endocervical invasion, LVI, serosal invasion, and MI were analyzed for OS and DFS.
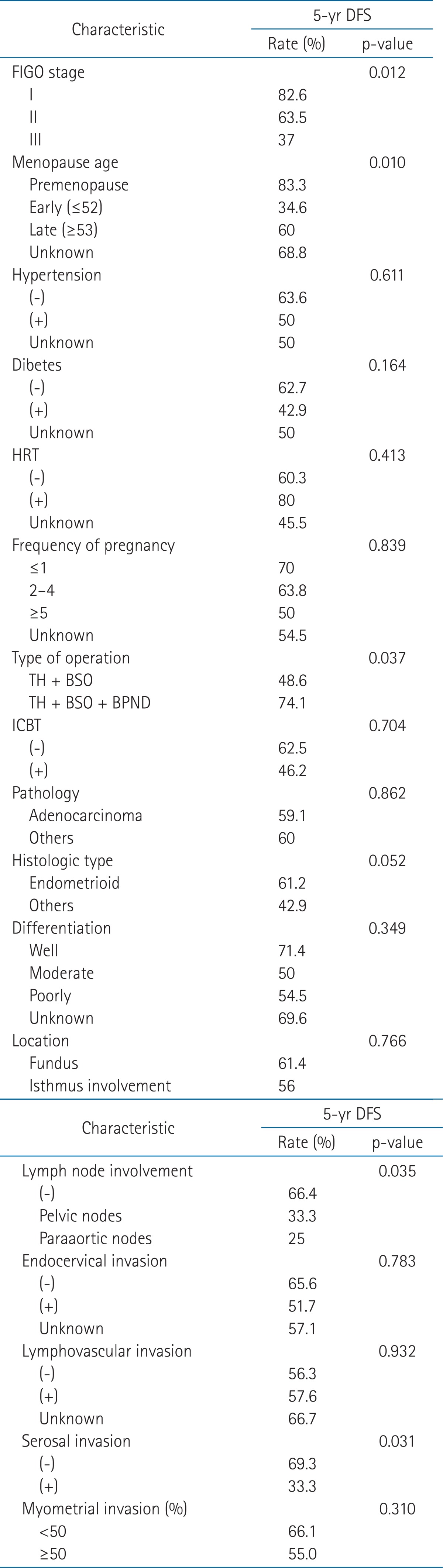
In univariate analysis, OS and DFS were significantly associated with the stage, menopausal age, type of operation, LN involvement and serosal invasion. Histologic type was marginally significant (Tables 2 and 3). The patients with higher stage had a significantly decreased OS and DFS than the patients with lower stage. The premenopausal and late menopausal patients had a significantly increased survival rate than the early menopausal patients. The patients with total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO) and bilateral pelvic node dissection (BPND) had a significantly increased OS and DFS when compared with the patients with TAH and BSO only. The histologic type was significantly related with OS, but was marginally significant with DFS. The endometrioid type had a significantly increased OS when compared with other histologic types. With respect to LN involvement in this analysis, all the patients were divided into 3 subgroups (no LN involvement, pelvic LN involvement, and paraaortic LN involvement). The 5-year OS rates were 65.8% in the group of no LN involvement, 33.3% in the group of pelvic LN involvement and 25% in the group of paraaortic LN involvement, respectively. Paraaortic LN involvement had decreased survival than pelvic LN involvement. Serosal invasion as well seemed to influence survival rate in the present study. The 5-year OS rates and DFS rates of serosal non-invasion group were 68.9% and 69.3%, respectively. In serosal invasion group, the 5-year OS rates and DFS rates were same as 33.3%. No other factors were statistically significant.
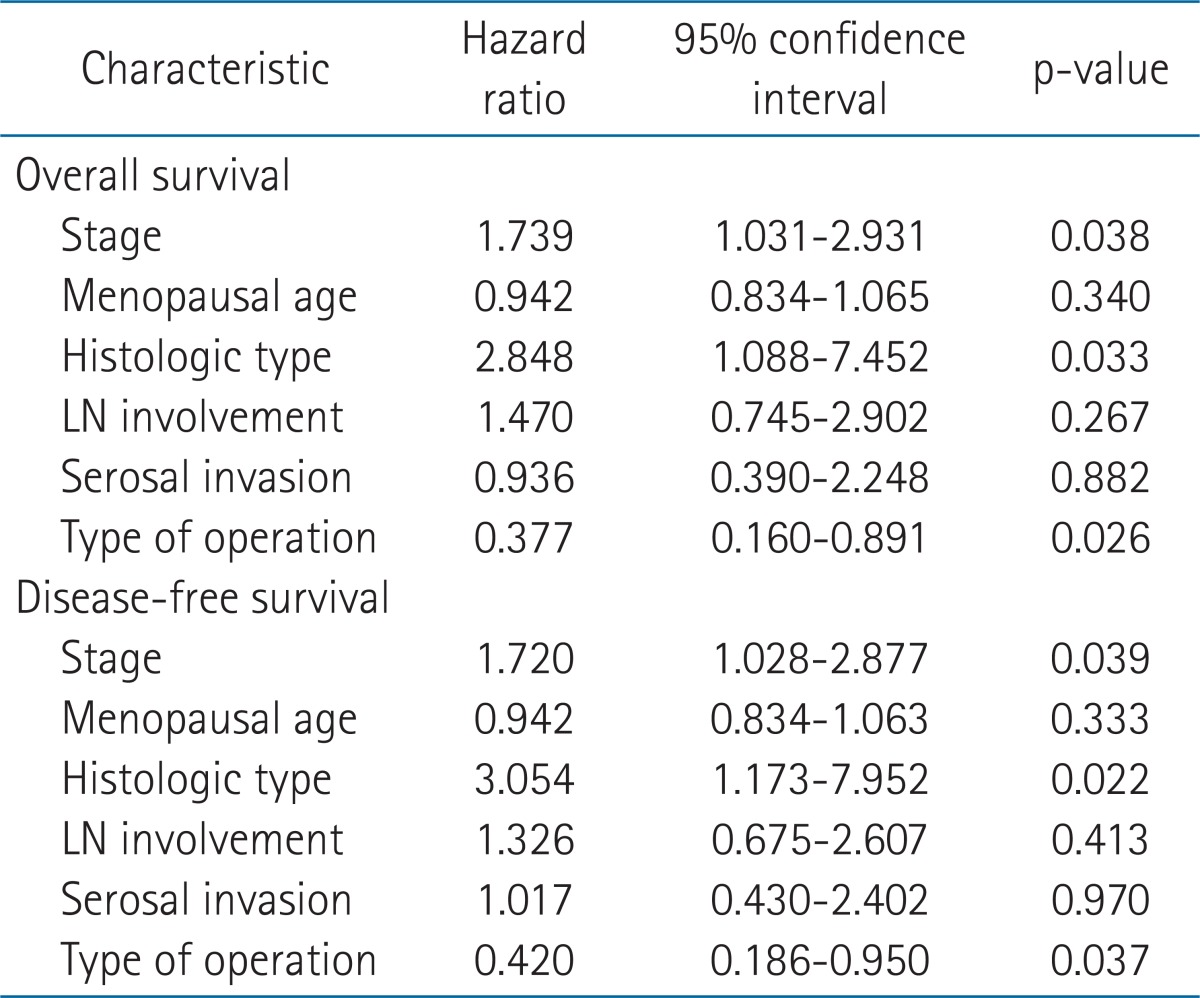
We analyzed some factors that had p-value of less than 0.1 in univariate analysis. In multivariate analysis, survival was significantly associated with the stage, type of operation and histologic type (Table 4).
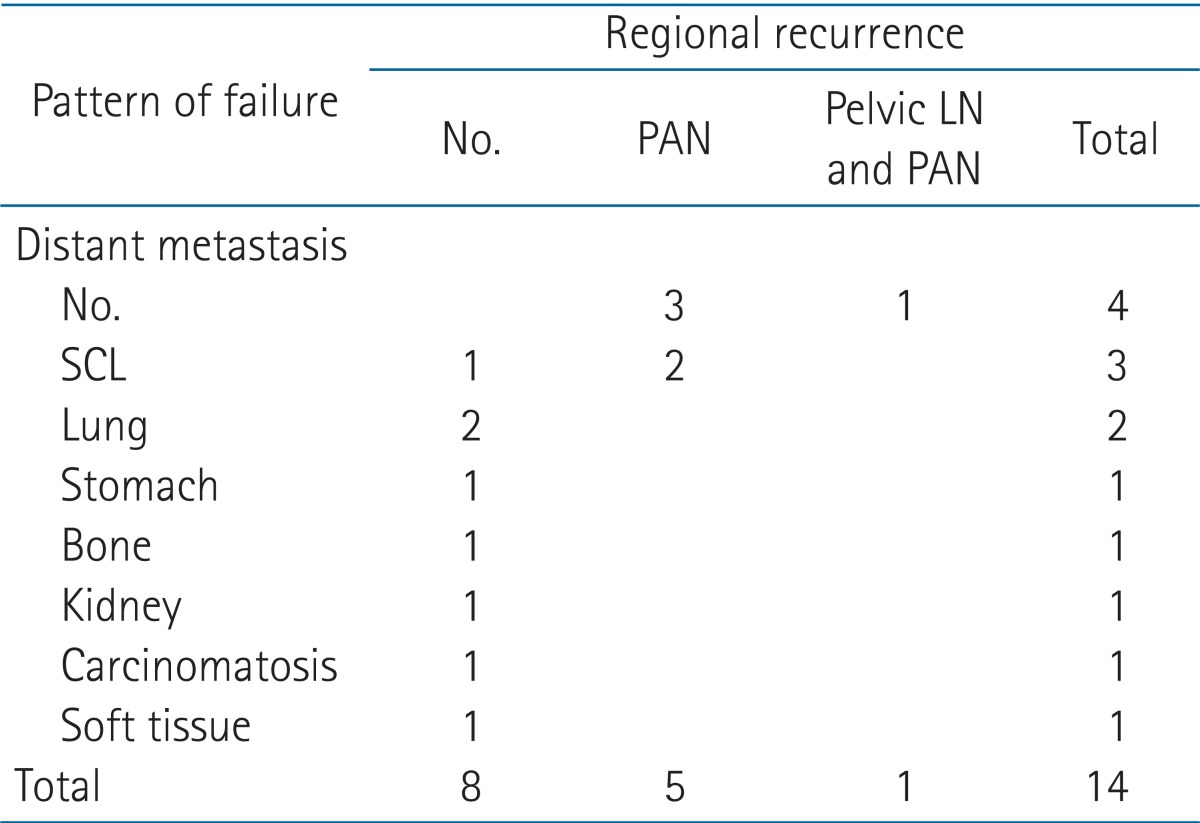
3. Failure patterns
Treatment failure occurred in 14 patients, regional recurrence occurred in 4 patients and distant metastasis occurred in 8 patients. Both of regional recurrence and distant metastasis occurred in 2 patients (Table 5). Of 4 patients with regional recurrence, 3 had paraaortic LN recurrences and 1 had both of pelvic LN recurrence and paraaortic LN recurrence. There was no vaginal recurrence. Of 8 patients with distant metastasis, 3 patients had supraclavicular LN metastasis and other 5 patients had distant organ metastases at various sites. The sites of distant metastases were lung, bone, kidney, stomach, soft tissue, and carcinomatosis peritonei. Two patients with both regional recurrence and distant metastasis had paraaortic LN and supraclavicular LN metastasis. Time to regional recurrence was from 4 to 21 months (median, 10.5 months) and distant metastasis was from 3 to 86 months (median, 12 months). Therefore, distant metastasis was considered as the predominant pattern of failure.
From an operative point of view, among 27 patients with BPND, distant metastasis occurred in 4 patients. Of 37 patients without BPND, regional recurrence occurred in 4 patients, distant metastasis occurred in 2 patients and both of regional recurrence and distant metastasis occurred in 2 patients.
Among 6 patients with paraaortic LN recurrences, 2 patients were treated with ART at paraaortic region and they were treated with chemotherapy for salvage treatment. Four patients were not treated with ART at paraaortic region. Two patients were treated with salvage radiotherapy and supportive management was only performed in two patients. The RT at supraclavicular region was performed on patients with supraclavicular metastasis. One patient with bone metastasis was treated with etoposide and cisplatin chemotherapy. Other patients with organ metastases were treated with supportive management.
4. Complications
There were no above grade 3 complications. Eleven patients had acute complications. Six patients had gastrointestinal (GI) complications such as nausea, vomiting, diarrhea and constipation and 5 patients had genitorurinary (GU) complications such as dysuria and incontinence. Eight patients had late complications. Five patients had GI complications such as rectal bleeding and mucoid stool and 3 patients had GU complications such as dysuria and hematuria.
Discussion and Conclusion
Previously, the role of EBRT for EC has been investigated in randomized and retrospective series, and it is still being debated [3-9].
Postoperative radiotherapy endometrial cancer-1 was a large randomized trial on 715 patients treated with standard surgery. The trial included patients with grade 2 (G2) EC, G1 EC with more than half of MI and G3 EC with less than half of MI. Whole pelvis radiotherapy was indicated in selected patients, with high-risk features of locoregional relapse including more than 60 years old, G1 and G2 EC with more than half of MI and G3 EC. It has been reported that there were significant locoregional control benefit in RT arm (5% vs. 14%, p < 0.001). There was no significant survival difference between RT arm and control arm (66% vs. 73%, p = 0.09) [4,5].
In 2004, the Gynecologic Oncology Group (GOG) reported a phase III trial as GOG 99 trial. Three hundred ninety two patients defined as MI, adenocarcinoma of any grade and no lymph node involvement, were treated with TAH/BSO plus lymphadenectomy and pelvic EBRT versus no-EBRT. Recurrence hazard decreased among EBRT treated patients when compared to those not treated with EBRT (relative hazard [RH], 0.42; 90% confidence interval [CI], 0.25 to 0.73; p = 0.007). A slight difference in the survival was observed between the two arms, though the difference was not statistically significant. The researchers analyzed the subgroups of intermediate risk. A subgroup of intermediate risk EC was defined to have risk factors such as increasing age, moderate to poorly differentiated, LVI and outer third MI. Patients about 50 years old with any 2 risk factors listed above, at least 70 years old with any risk factor listed above or any age with more than three risk factors listed above were also defined as high intermediate risk. It was demonstrated that EBRT significantly reduced the risk of recurrence in high intermediate subgroup (RH, 0.42; 90% CI, 0.21 to 0.83) [6].
In a study conducted by Aalders et al. [7], 540 patients received EBRT or no-EBRT after primary surgery for stage I adenocarcinoma of the uterus. Two hundred seventy seven patients received no other treatment, and 263 patients underwent EBRT. A reduction in vaginal and pelvic recurrences was found in the EBRT group (1.9% vs. 6.9%, p < 0.01). However, there was no difference in 5-year OS (89% vs. 91%) rate in both the groups. It was only in the subgroup of poorly differentiated tumors, infiltrating more than half of the myometrial thickness, where a survival benefit was suggested [7].
Our results in DFS rates, Locoregional recurrence (LR) and DM are consistent with several retrospective and prospective studies, which showed 5-year DFS rates of about 83% and relapse rates of about 20%, of which 6% are LR and 15% are DM [7]. Distant metastasis was found to be predominant failure pattern in several studies including our study. Therefore, we contemplated that the other adjuvant treatment would be needed to reduce distant metastasis.
In several clinical trials, concurrent chemoradiotherapy has been performed on patients with EC, who had high risks such as more than stage III, positive paraaortic LN or more than half MI [10-14].
Randall et al. [10] reported the results of the GOG 122 including 396 patients with residual disease smaller than 2 cm in stage III and IV EC after staging operation. Patients received either postoperative whole abdominal irradiation (WAI) or chemotherapy with cisplatin and doxorubicin (AP) and chemotherapy was found to have survival benefit. AP resulted in progression hazard ratio (HR) of 0.71 (p = 0.007) and death HR of 0.68 (p = 0.004) compared to WAI. Five-year progression-free survival (PFS) rate of AP and WAI was 50% and 38%, OS rate was 55% and 42%, respectively.
According to the European Organisation for Research and Treatment of Cancer (EORTC) 55991 trial, 372 patients with FIGO stage I, II, IIIA, or IIIC disease were included. In this study, chemotherapy was given before or after radiotherapy. Chemotherapy consisted of four courses, including cisplatin plus doxorubicin, cisplatin plus epirubicin, paclitaxel plus epirubicin plus carboplatin, or paclitaxel plus carboplatin. The HR for PFS was 0.58 in group of radiotherapy plus chemotherapy (p = 0.046). Five-year PFS rate was 75% in radiotherapy and 82% in radiotherapy plus chemotherapy groups [14].
We could not carry out further investigation on chemotherapy after or before radiotherapy, as this study was performed retrospectively, and fewer patients were treated with concurrent chemoradiotherapy (CCRT) in our institute. However, we contemplated that chemotherapy could reduce the progression of disease in EC. In advanced EC, chemotherapy would be beneficial for OS and PFS. After several years, additional studies would be required to understand effectiveness of chemoradiotherapy.
Some randomized trials about postoperative radiotherapy in EC, were going on. Postoperative radiotherapy EC-3 investigators are testing the difference of postoperative radiotherapy versus CCRT followed by adjuvant carboplatin plus paclitaxel for high-risk stage IB-III EC. Eligibility patients include stage IB/G3 with LVI, stage IC-IIA/G3, more than stage IIB and serous or clear cell carcinoma. GOG 258 trial is a phase III trial of cisplatin and RT. Followed by carboplatin and paclitaxel versus carboplatin and paclitaxel chemotherapy for stage III and IV advanced EC. GOG 249 trial is also phase III trial of pelvic RT versus vaginal brachytherapy followed by paclitaxel and carboplatin in patients with high risk stage I and II endometrial cancer. Eligibility patients include stage I with risk factors such as G2-3, LVI and more than half MI, and stage II EC. Total hysterectomy plus BSO was performed on them [15].
Over the past few decades, several studies have reported the prognostic factors for survival including LN involvement, histological type, grade, stage, MI, LVI and cervical invasion [16-21].
Irwin et al. [16] reported the results of retrospective study carried out on 550 patients with stage I EC. Patients received EBRT, EBRT plus ICBT or ICBT after surgery. According to the results of this study, FIGO grade (p = 0.009), lower uterine segment involvement (p = 0.009) and age (p = 0.03) were considered to be significant predictors of worse DFS in multiple regression analysis. In a study conducted by Uharcek [17], other factors which were investigated included estrogen and progesterone receptor status and p53 status. Yalman et al. [18] reported the retrospective analysis carried out on 440 patients with EC treated by postoperative radiotherapy. According to multivariate analysis, prognostic factors for DFS were histologic type (p < 0.02), MI (p = 0.0021) and histologic grade (p = 0.03). The MI was the only prognostic factor for local recurrence-free survival.
In the present results of univariate analysis, stage, menopausal age, type of operation, histologic type, LN involvement and serosal invasion were associated with OS and DFS. In multivariate analysis, stage, histologic type and types of operation could be the plausible factors to predict prognosis of patients. In past studies, menopausal age was not estimated as prognostic factor for survival. In our study, patients with early menopause had significantly decreased OS and DFS in univariate analysis. In recent opinion based on several studies, late menopause has been found to be associated with the development of EC. Late menopause may reflect prolonged exposure to unopposed estrogen stimulation. Unopposed estrogens, from either endogenous or exogenous sources, stimulate abnormal proliferation of endometrial cells, including malignant transformation [22,23]. Because of this effect of unopposed estrogen, early menopausal age could be the possible prognostic factor for OS and DFS. Additional incessant studies would be needed in future.
In conclusion, postoperative radiation was found to be the effective and safe treatment method for EC. However, adjuvant treatment would be needed to reduce distant metastasis because distant metastasis was the cause for predominant pattern of failure. Stage, histologic type and types of operation were significant prognostic factors. Menopausal age could have the possibility of serving as a prognostic factor. Therefore, additional prospective or retrospective studies should be designed and carried out in an incessant manner.
Notes
No potential conflict of interest relevant to this article was reported.