Concurrent chemoradiotherapy for elderly patients with stage III non-small cell lung cancer
Article information
Abstract
Purpose
Combined chemoradiotherapy is standard management for locally advanced non-small cell lung cancer (LA-NSCLC), but standard treatment for elderly patients with LA-NSCLC has not been confirmed yet. We evaluated the feasibility and efficacy of concurrent chemoradiotherapy (CCRT) for elderly patients with LA-NSCLC.
Materials and Methods
Among patients older than 65 years with LA-NSCLC, 36 patients, who underwent CCRT were retrospectively analyzed. Chemotherapy was administered 3-5 times with 4 weeks interval during radiotherapy. Thoracic radiotherapy was delivered to the primary mass and regional lymph nodes. Total dose of 54-59.4 Gy (median, 59.4 Gy) in daily 1.8 Gy fractions and 5 fractions per week.
Results
Regarding the response to treatment, complete response, partial response, and no response were shown in 16.7%, 66.7%, and 13.9%, respectively. The 1- and 2-year overall survival (OS) rates were 58.2% and 31.2%, respectively, and the median survival was 15 months. The 1- and 2-year progression-free survivals (PFS) were 41.2% and 19.5%, respectively, and the median PFS was 10 months. Regarding to the toxicity developed after CCRT, pneumonitis and esophagitis with grade 3 or higher were observed in 13.9% (5 patients) and 11.1% (4 patients), respectively. Treatment-related death was not observed.
Conclusion
The treatment-related toxicity as esophagitis and pneumonitis were noticeably lower when was compared with the previously reported results, and the survival rate was higher than radiotherapy alone. The results indicate that CCRT is an effective in terms of survival and treatment related toxicity for elderly patients over 65 years old with LA-NSCLC.
Introduction
As aging population is increased, old age is important risk factor on the tumor. More than 50% of all lung cancers are diagnosed in patients with age >65 years, and approximately 30% are diagnosed in patients with age >70 years [1,2]. Non-small cell lung cancer (NSCLC) is the most common type which represents more than 80% of lung cancer [3]. Lung cancer is the leading cause of cancer mortality throughout the world, including Koreas [4].
Until the mid-1980s, thoracic radiotherapy had been regarded as the standard treatment for locally advanced non-small cell lung cancer (LA-NSCLC), but results of thoracic radiotherapies were disappointing, as 70% of patients showed distant metastasis, durable local control was less than 20%, median survival was less than 1 year, and long-term survival rates of 3-7% [5]. These poor results were largely involved as introducing multimodality therapy. We obtained knowledge of multimodality therapy is beneficial to NSCLC from the result of treatment with cisplatin based chemoradiotherapy which was superior than radiotherapy alone, and concurrent chemoradiotherapy (CCRT) was superior than induction chemotherapy followed by radiotherapy alone [6,7].
The treatment outcomes of the studies considered the effect of the age were varied. In addition, studies on the usefulness of aggressive combined-modality treatment for elderly patients with LA-NSCLC contradict each other. Generally, benefit in overall survival (OS) accompanies with toxicity increment. It is very important to assess whether old patients could withstand combined-modality therapy as much as young patients and whether to gain the benefit from treatment. There are 2 primary reasons that can't directly apply the results of CCRT for elderly patients with LA-NSCLC [8]. One is proportion of elderly patients were less in these clinical studies than proportion in general population, and the other one is multimodality therapy increases treatment-related toxicity. Therefore, the present study was tried to evaluate the feasibility and efficacy of CCRT for elderly patients with LA-NSCLC.
Materials and Methods
From March 2003 to December 2010, among 43 patients who were older than 65 years were eligible if they had; histologically diagnosed with the American Joint Committee on Cancer (AJCC) stage IIIA or IIIB NSCLC; Eastern Cooperative Oncology Group (ECOG) performance status 0, 1 or 2; not previously treated with chemotherapy or radiotherapy; adequate bone marrow function, normal renal function, normal hepatic function, and normal pulmonary function, excluding 5 patients who had not completed the schedule radiotherapy and 2 patients who gave up the treatment voluntarily, the study was performed on 36 patients who were treated with CCRT. Thirteen (36.1%) patients were stage IIIA, and 23 (63.9%) patients were stage IIIB. The records of the patients and their diseases were reviewed retrospectively. The outcomes of treatment were analyzed in terms of survival rates, and treatment related complications.
All patients were treated with CCRT. For the radiotherapy, thoracic radiotherapy techniques were applied using Clinac 21EX (Varian Medical Systems, Palo Alto, CA, USA). Total dose of radiation therapy 54-59.4 Gy (median, 59.4 Gy) was delivered with daily 1.8 Gy per fraction and five fractions a week. The lesion found by a chest computed tomography (CT) or positron emission tomography (PET)/CT was defined as gross target volume (GTV). Then the clinical target volume (CTV) was defined the GTV with a 1.0-1.5 cm margin plus the area including the regional lymph node (LN), and the planning target volume (PTV) was the CTV with 0.5-1.0 cm margin. For the initial treatment, up to 36 Gy was delivered using the anteroposterior/posteroanterior (AP/PA) apposing fields, and the remained treatments were completed with 3-demensional conformal radiotherapy. Chemotherapy was initiated simultaneously with radiotherapy. Paclitaxel alone or the combination of paclitaxel and cisplatin was administered with 4-week intervals for 3-4 times. A dose of 60 mg/m2 of paclitaxel was delivered three times with 1-week intervals (days 1, 8, and 15) for 4 weeks. A dose of 25 mg/m2 of cisplatin was intravenously injected 3 times (days 1, 8, and 15) for 4 weeks. Two times was concurrently administered with radiotherapy and 1 to 2 times was administered adjuvant treatment.
The treatment results were assessed via imaging tests that were performed after 4-6 weeks from the completion of the treatments. The response to the treatment was evaluated according to the World Health Organization (WHO) criteria. Complete response (CR) was defined as complete disappearance of the lesions, and partial response (PR), when the lesion shrank by more than half. Stable disease (SD) was those with sizes between 50% and 125% of their previous size. Progressive disease (PD) was defined as those whose size increased by more than 125% or developed extra lesion. With regard to the complications induced by the treatment, radiation-induced lung toxicity was evaluated with the Radiation Therapy Oncology Group (RTOG) grading system, and other complications were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE, v4.0).
OS was defined as the elapsed time from the initial treatment date to the death or to the patient's last visit. Progression-free survival (PFS) was defined as the time from the initial treatment date to the date of disease progression or the date of the patient's last visit. The initial recurrence was categorized as locoregional recurrence (LRR) or distant metastasis (DM) depending on the location of the recurred lesion. Statistical analysis was performed using SAS ver. 6.02 (SAS Institute, Cary, NC, USA). PFS and OS were analyzed using the Kaplan-Meier survival method. The p-values were calculated using the log-rank test, and the cases with a p-value less than 0.05 were evaluated as statistically significant.
Results
1. Characteristics of patient
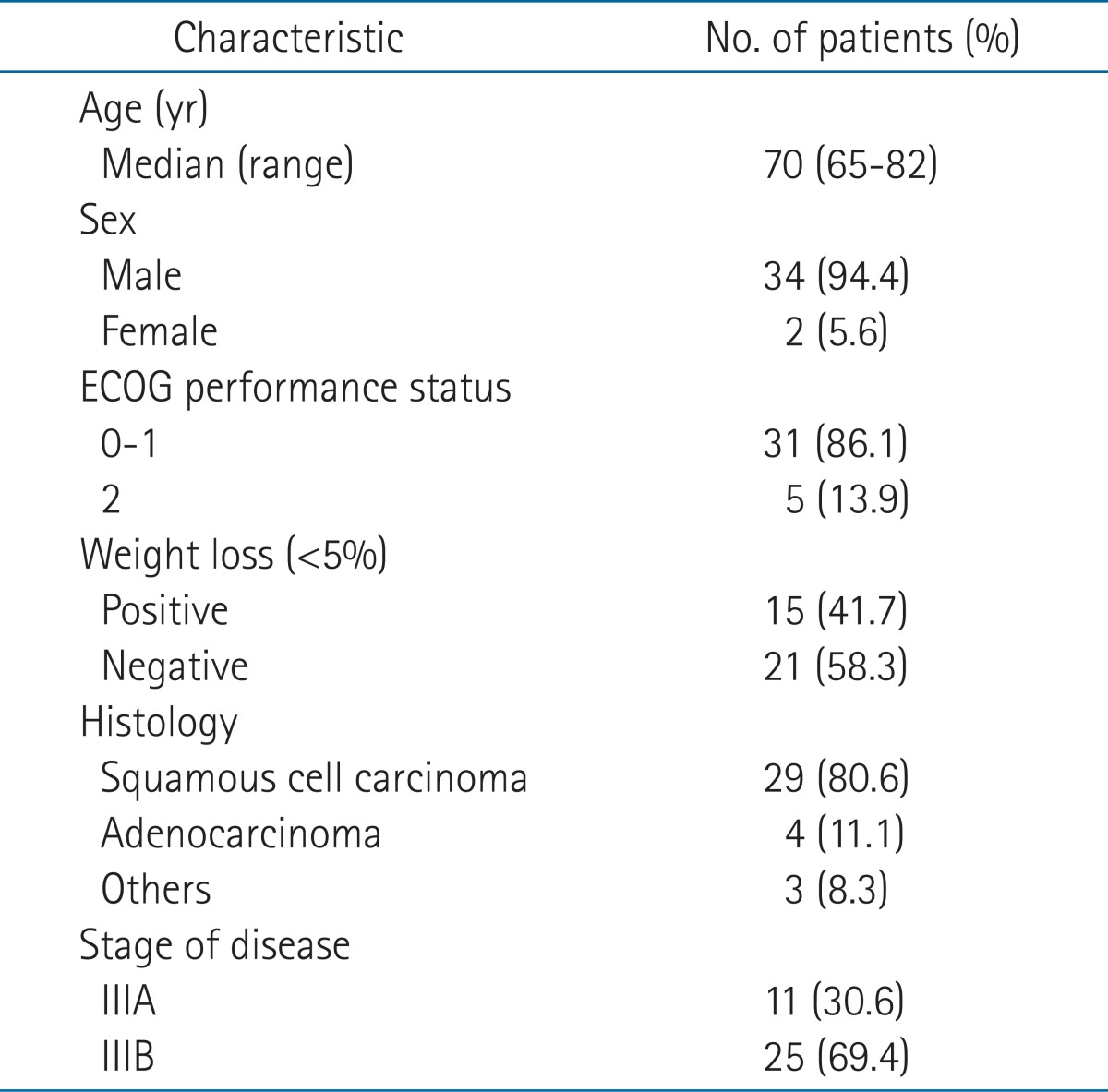
The characteristics of the patients are summarized in Table 1. The mean age of patients was 70 years (range, 65 to 82 years). Thirty-four (94.4%) were male and 2 (5.6%) patients, female. The performance status of 31 patients was 0-1 (86.1%), and of 5 patients, 2 (13.9%). Their performance status was relatively well. Fifteen (41.7%) patients had lost weight at the time of diagnosis. With regard to their pathological classification, 29 (80.6%) patients had squamous cell carcinoma and only 4 (11.1%) patients had adenocarcinoma. At the time of diagnosis, the disease stage of 11 (30.6%) patients was IIIA, and of 25 (69.4%) patients, IIIB. The follow-up duration was 4 to 62 months, and the median follow-up period was 18 months.
2. Response to treatment
Table 2 summarizes the outcome of the CCRT. The percentages of the patients with a complete response, partial response, stable disease, and progressive disease were 6 (16.7%) patients, 24 (66.7%) patients, 5 (13.9%) patients, and 1 (2.8%) patient, respectively. The rate of overall response to the CCRT was 30 (83.3%) patients. Table 3 shows the recurrence patterns of the patients. LRR occurred in 10 (27.8%) patients; distant metastasis, in 9 (25.0%) patients; and both LRR and DM, in 1 (2.8%) patient.
3. Survival
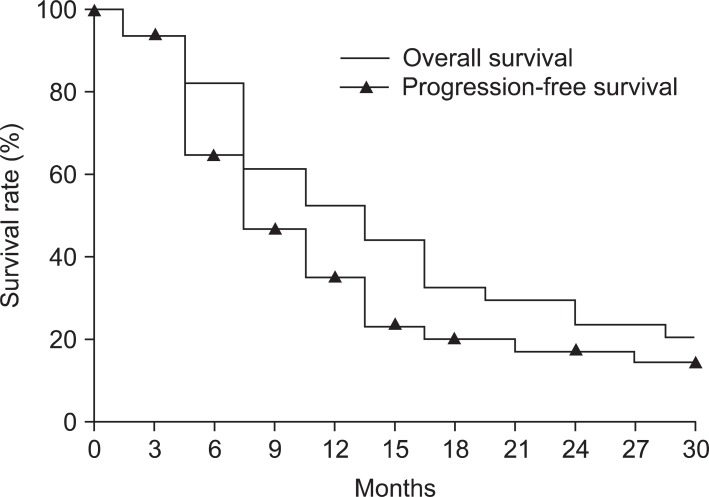
The median follow-up duration in the entire population was 18 months (range, 4 to 62 months). Fig. 1 shows the OS and the PFS rates. The 1- and 2-year OS rates of the treated patients were 58.2% and 31.2%, respectively, and the duration of median survival was 15 months. The 1- and 2-year PFS rates were 41.2% and 19.5%, respectively, and the duration of median PFS was 10 months.
4. Toxicity caused by treatment
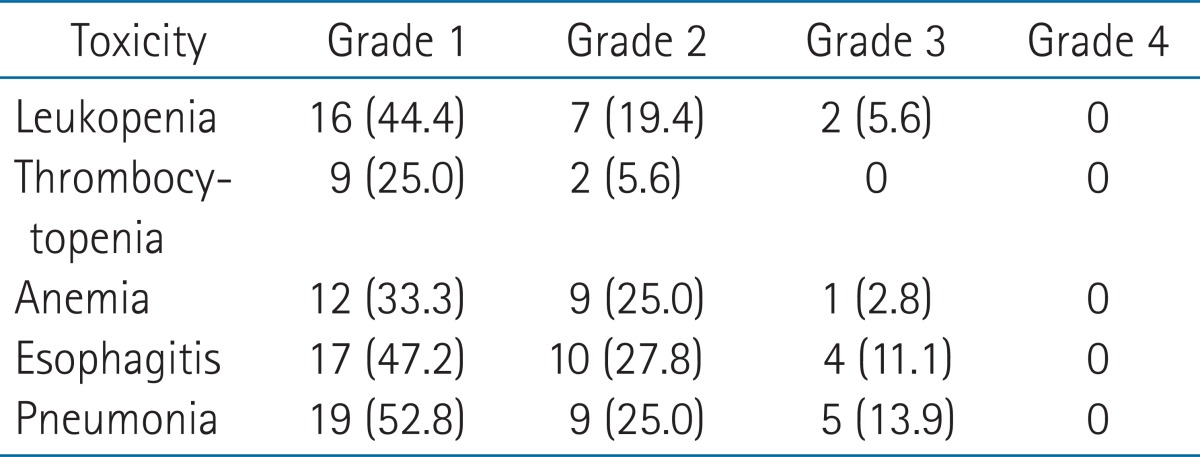
Table 4 shows the toxicity related with CCRT. Various complications such as leukopenia, thrombocytopenia, anemia, esophagitis, and pneumonia were developed, but severe complication with grade 4 or higher were not observed. The grade 3 leukopenia, anemia, esophagitis, and pneumonia were observed in 2 (5.6%) patients, 1 (2.8%) patient, 4 (11.1%) patients, and 5 (13.9%) patients, respectively. None of the patients died due to the treatment during the treatment and follow-up periods.
Discussion and Conclusion
The clinical benefit of CCRT on the LA-NSCLC over the definitive radiotherapy alone was supported by some clinical reports. For example, prospective randomized clinical trial by Atagi et al. [9], which analyzed 200 patients older than 70 years with LA-NSCLC. The main conclusion in the study was that the combination chemoradiotherapy provides a clinically significant benefit over radiotherapy alone. The median OS for the chemoradiotherapy and radiotherapy alone group were 22.4 and 16.9 months, respectively (p = 0.018). However, the clinical gain of combined multimodality therapy has become largely controversial when specified to elderly patients. This controversy was mostly due to difference in the results for the treatment-induced complications. Some clinical reports argued that the aggressive combined therapy was tolerable even in elderly patients, while others stated that the complication risk might exceed the clinical benefit.
The phase II clinical trials of Southwest Oncology Group by Lau et al. [10], which prospectively analyzed 60 patients with stage III NSCLC, basically argued that the aggressive CCRT was tolerable and beneficial even for the patients with poor prognostic factors. This argument was supported by Davidoff et al. [11] who reported the clinical gain of combined modality therapy with the comparable median survival (10.4 months) and 2-year survival rates (23.0%) with those in the phase II clinical trials by Southwest Oncology Group (13.0 months and 21.0%, respectively) [10]. Moreover, Kang et al. [12] reported that induction chemotherapy with paclitaxel and cisplatin followed by CCRT for stage IIIB NSCLC yielded more improved clinical outcomes, where overall response rate made up 51.3% and 1-, 2-, and 3-year survival rates amounted to 66.7%, 40.6%, and 27.4%, respectively.
In contrast, much more clinical studies raised negative results on this issue. For example, Hayakawa et al. [13] recommended definitive radiotherapy, instead of CCRT, based on their evaluation of high-dose radiotherapy elderly inoperable NSCLC patients. The RTOG [14,15] was also suggested, for patient with age more than 70 years, radiotherapy alone was more appropriate due to the increased risk of complication. Concerns over the increased complication risk for elderly patients also can be seen in the report for North Central Cancer Treatment Group experience by Schild et al. [16], where survival rate was not much changed with the patient's age but the incidence rate for severe complications was significantly increased with patient's age; for the patient groups younger and lower than 70 years, 2-year and 5-year survivals were 39% and 18% vs. 36% and 13%, while 4 or higher pneumonitis and hematologic toxicity were 1% and 56% vs. 6% and 78%. Based on the results, this report also concluded that the radiotherapy alone should be considered as the first choice for elderly patients to avoid severe complications.
Because of this sharp contrast with slightly biased to the negative results, it seems that most of institutions prefer to the safe course (radiotherapy alone) rather than the aggressive one (CCRT) for the treatments of elderly patients with LA-NSCLC. However, our clinical results strongly suggested that the aggressive CCRT could be also effective and safe in elderly patient group in considering of both respects of survival gain as well as complication risk. The treatment response in the present study amounted to 6 (16.7%) patients for CR and 24 (66.7%) patients for PR. The overall response rate was 30 (83.3%) patients, which was substantially higher than any of the previously reported results (e.g., 29% in Southwest Oncology Group [10] and 51.3% in Kang et al. [12]). The median survival (15.0 months) in our results was also better than previous results (13.0 months in Southwest Oncology Group [10] and 10.4 months in Davidoff et al. [11]) even though the present results only included the elderly patients with ages more than 65 years. The 2-year OS rates of 19.5% was comparable, than the previous results (21.0% in Southwest Oncology Group [10], 23.0% in Davidoff et al. [11]). These results showed that benefit of multimodality therapy in tumor control can be preserved in elderly patients. In addition, the treatment-induced complication, which might be the most critical point when considering the CCRT to elderly patients, was sharply lower than the previous results. Unlike the previous report by Schild et al. [16], reported grade 4 or higher hematologic toxicity were 56% in younger than 70 years while 78% in older than 70 years, grade 4 or higher pneumonitis were 1% and 6%, respectively. In this study, higher than grade 3 leukopenia, anemia, esophagitis, and pneumonia were developed only in 2.8-13.9% of patients, which was lower than clinical report by Kang et al. [12] with grade 3 or higher pneumonitis and esophagitis in 15-78% of patients.
In conclusion, the present result for CCRT to elderly patients LA-NSCLC yielded very satisfactory clinical outcomes not only in the survival gain but also in the treatment-related toxicity. The complication rate was noticeably reduced and the developed complications were relatively mild with the present CCRT regimen. The OS and PFS rates in this study were also superior to those in radiotherapy alone and those in other CCRT results. Even if it is hard to guarantee, the reason for these results is to use the developed highly conformal radiotherapy technique and improved efficacy of the new chemotherapy agent for younger patients than other studies and another reason is selection bias about higher performance status patients were selected for this study than other studies. This study has limitations to be generalized because only 36 patients were analyzed, and has selection bias due to retrospectively analyzed. Nevertheless, considering the very good clinical outcomes shown in this study, it is worthwhile to apply the present CCRT regimen to the elderly patients for LA-NSCLC management.
Notes
No potential conflict of interest relevant to this article was reported.