Postoperative chemoradiotherapy in high risk locally advanced gastric cancer
Article information
Abstract
Purpose
To evaluate treatment outcome of patients with high risk locally advanced gastric cancer after postoperative chemoradiotherapy.
Materials and Methods
Between May 2003 and May 2012, thirteen patients who underwent postoperative chemoradiotherapy for gastric cancer with resection margin involvement or adjacent structure invasion were retrospectively analyzed. Concurrent chemotherapy was administered in 10 patients. Median dose of radiation was 50.4 Gy (range, 45 to 55.8 Gy).
Results
The median follow-up duration for surviving patients was 48 months (range, 5 to 108 months). The 5-year overall survival rate was 42% and the 5-year disease-free survival rate was 28%. Major pattern of failure was peritoneal seeding with 46%. Locoregional recurrence was reported in only one patient. Grade 2 or higher gastrointestinal toxicity occurred in 54% of the patients. However, there was only one patient with higher than grade 3 toxicity.
Conclusion
Despite reported suggested role of adjuvant radiotherapy with combination chemotherapy in gastric cancer, only very small portion of the patients underwent the treatment. Results from this study show that postoperative chemoradiotherapy provided excellent locoregional control with acceptable and manageable treatment related toxicity in patients with high risk locally advanced gastric cancer. Thus, postoperative chemoradiotherapy may improve treatment result in terms of locoregional control in these high risk patients. However, as these findings are based on small series, validation with larger cohort is suggested.
Introduction
Gastric cancer is the most frequent cancer in Korea [1]. Surgical resection is essential for cure in these patients. However, substantial recurrence rates have been reported after surgical resection alone and adjuvant treatment showed survival benefit in several studies [2-4]. Despite agreement in need for adjuvant therapy, optimal adjuvant regimen is still controversial. In United States, large phase III trial, the intergroup 0116 [4], showed survival benefit with postoperative chemoradiotherapy (CRT) and CRT is widely accepted as a component of standard of care. In contrast, adjuvant chemotherapy without radiotherapy is more frequently used in Korea. Publications [5,6] reporting uncertain benefit of postoperative radiotherapy (PORT) in patients after D2 lymph node dissection was underlying rationale for omitting PORT. In our institution, PORT was reserved for high risk advanced cases after surgical resection; margin involvement or adjacent structures invasion. Herein is an analysis of treatment results of these patients.
Materials and Methods
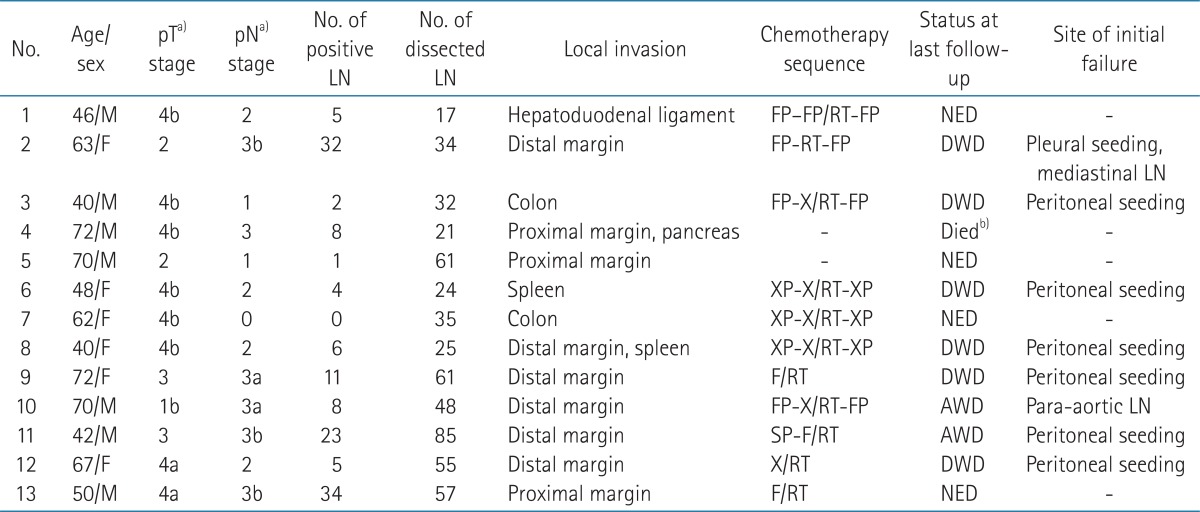
During May 2003 to May 2012, sixteen patients with gastric cancer received PORT. Two patients with recurrent diseases after primary resection and one patient with squamous cell carcinoma histology were excluded from analysis. Thus, 13 patients with gastric adenocarcinoma were included for analysis. Seven patients were male and 6 patients were female. Median age at surgical resection was 62 years (range, 40 to 72 years). Selected patients with resection margin (RM) involvement and/or adjacent organ invasion were referred for PORT per attending surgeon or medical oncologist's discretion. Six patients had involved distal RM; 3 patients had proximal RM involvement. Six patients had invasion to other organs (colon in two, spleen in one, and hepatoduodenal ligament in one). One patient had tumor with proximal margin involvement and pancreatic invasion and the other patient had tumor with distal margin involvement and spleen invasion. Tumor was located in the upper third of stomach for 10 (77%) patients. Eight patients underwent total gastrectomy, 3 patients subtotal gastrectomy, another patient extended total gastrectomy, and the other patient received pancreaticoduodenectomy. Ten (77%) patients underwent D2 nodal dissection. Median number of dissected lymph node was 35 (range, 17 to 85). Lymph node metastases were observed in all but one patient (92%). Median number of involved lymph node was 7 (range, 1 to 34). Patients were staged according to the American Joint Committee on Cancer Staging, 7th edition.
Adjuvant chemotherapy was used in 11 (85%) patients. Of these patients, 10 (77%) patients were treated with concurrent CRT. Chemotherapy regimen concurrent with radiotherapy was capecitabine for 5 patients, 5-flurouracil (5-FU) for 3 patients, and 5-FU with cisplatin for 2 patients. Two patients did not receive adjuvant chemotherapy due to poor performance. CRT or PORT was given between adjuvant chemotherapy cycles (sandwich schedule) in 7 patients, after chemotherapy in one patient, and before chemotherapy in one patient. Other 2 patients received CRT only. Radiotherapy was targeted to the tumor bed and regional lymphatics with exception of splenic hilar node with computed tomography (CT) scan based treatment plan. Median total dose of PORT was 50.4 Gy (range, 45 to 55.8 Gy). The dose per fraction was 1.8 Gy in all patients. After 45 Gy, 8 (62%) patients received median 5.4 Gy (range, 5.4 to 10.8 Gy) boost to tumor bed. Dose distribution examples of patients with margin involvement are shown in Fig. 1. The details of patient characteristics are summarized in Table 1. After treatment completion, follow-up visits were scheduled at every 3 months. Abdomen CT scans were performed every 3 months in first year, then every 6 months during the next two years and yearly thereafter.

Dose distribution examples of (A) distal margin involved and (B) proximal margin involved patient. Darker shade in each figure indicates reduced planning target volume for respective margin involvement.
Results
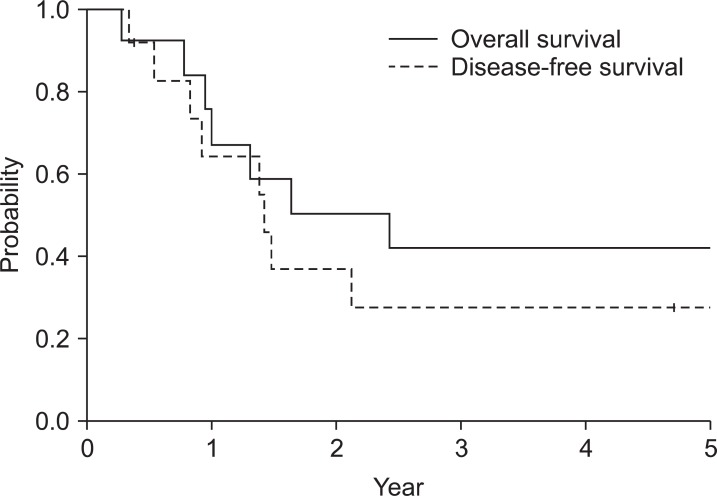
Median follow-up duration of surviving patients was 48 months (range, 5 to 108 months). Fig. 2 shows the survival curves for overall survival and disease-free survival. The 5-year overall survival rate was 42%. Cause of death was disease recurrence except one patient who died 4 days after completion of PORT with unknown cause. The 5-year disease-free survival rate was 28%. Two patients were alive with recurrence at the last follow-up. Follow-up durations of these patients were 13 and 18 months. In contrast, median time to death from recurrence was 4 months in patients who succumbed to disease. Distant failure was initial site of failure for all recurred patients. Six (46%) patients initially recurred with peritoneal seeding, another patient with para-aortic lymph node metastasis and the other patient with pleural seeding. Local recurrence was noted in a patient at remnant stomach who had initial margin involvement 11 months after para-aortic lymph node relapse.
In terms of margin involvement, 5-year overall survival rates of margin negative and positive patients were 50% and 38%, respectively. Recurrence rate and local control rate of margin negative and positive group were 50%, 67% and 100%, 89%, respectively. There was no significant difference in overall survival, disease-free survival, and local recurrence-free survival between patients who received PORT due to adjacent organ invasion and positive RM (p = 0.686, 0.432, 0.317, respectively). Univariate analysis was performed with log-rank test. Among various clinical and pathological parameters, upper stomach location was only factor associated with poor prognosis (p = 0.011). Additional multivariate analysis was not done due to limited number of patient and event.
Treatment morbidities were graded according to Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0. Grade 3 or higher acute hematologic toxicity was reported in one patient with grade 3 anemia. Seven (54%) patients experienced grade 2 or greater acute gastrointestinal (GI) toxicity. However, there was only one patient with grade 3 GI toxicity, which was emesis. Six (46%) patients suffered nausea, 6 patients (46%) anorexia, 5 patients (38%) esophagitis, and one patient (8%) diarrhea. Chronic morbidity was reported in one patient, which was portal vein thrombosis.
Discussion and Conclusion
Optimal adjuvant treatment for gastric cancer in form of either perioperative chemotherapy or CRT is still controversial. Large prospective randomized study, namely Intergroup Study 0116 [4], which randomized 556 patients, showed clear benefit of CRT over observation. Besides, several studies [2,3] reported that adjuvant chemotherapy was beneficial. In contrast to adjuvant chemotherapy, role of PORT is relatively unsettled. Dutch study [5] showed little benefit from adjuvant CRT in D2 nodal dissection in contrast to D1 dissection. These findings were one of the underlying rationale for limited use of adjuvant CRT after D2 nodal dissection in clinical practice. Recently, the adjuvant chemoradiation therapy in stomach cancer (ARTIST) trial researchers also reported that addition of PORT was not significantly beneficial in terms of overall survival [6]. However, subgroup analysis of ARTIST trial revealed that patients with lymph node metastasis had prolonged survival with PORT. In spite of extensive surgery like D2 dissection and adjuvant chemotherapy, 8.3-23.3% of patients relapsed locoregionally without PORT [6,7]. These finding suggests that some proportion of patients would benefit from PORT. In our institution, selected patients with high risk locally advanced characteristics such as adjacent structures invasion or failed to obtain negative margin were referred for PORT. These strict indications explain why there was only limited number of patients during relatively extended accrual period. As patients were highly selected, most patients in current study had many high risk features. Nodal involvement was in 92% of the patients and 77% of patients were stage III. Despite high risk features, locoregional control was achieved in 92% of patients. These finding suggests that PORT with or without chemotherapy would contribute in obtaining excellent locoregional control even in these high risk locally advanced cases. However, high rate of distant failure especially peritoneal seeding in this advanced group inferred that substantial number of patients already had disease burden in peritoneal cavity. This in turn may limit the role of additional locoregional treatment modality, such as PORT.
There are only limited number of reports on the treatment results of adjuvant CRT after D2 nodal dissection, including two randomized trials [6,7] and several non-randomized single institution studies [8-11]. Treatment result of advanced stage patients comparable to this study was reported in two retrospective series. The 5-year survival rate of stage IIIB patients after PORT was 45% in a study by Lim et al. [8] and 50% in a study by Kim et al. [11], which is comparable to current series. Grade 3 or more GI toxicity rate was also similar with other studies (8% vs. 5-12%, retrospectively) [6-11]. In adjuvant chemotherapy alone trials [3,12] with D2 lymph node dissection, majority of patients had T2 or T3 disease and only 0% to 2.6% of patients had T4 disease. Despite use of adjuvant chemotherapy, peritoneum was the most common site of recurrence in both studies, partly reflecting the nature of this disease. The Polish Gastric Cancer Study Group conducted multicenter clinical trial [13] with 79% of patients receiving D2 lymph node dissection and 29% of patients having T4 tumor. Local recurrence rate was 16% and 5-year survival rate was 44% with adjuvant chemotherapy alone. In contrast, despite small number, present study showed less local recurrence rate (8%) with more T4 disease (54%). Sun et al. [14] reported 5-year survival rate of 25.8% and locoregional recurrence rate of 29.6% without adjuvant treatment for patients with margin involvement after surgery including D2 dissection.
However, findings from current series are drawn from observation of only 13 patients. Thus, in addition to inborn limitations of retrospective analysis, various statistically analyzed end-points are of limited value. The biggest pitfall maybe that some of results that are shown to be statistically insignificant may well be significant, if larger number of patient and in turn events are included for analysis.
In conclusion, despite reported suggested role of adjuvant radiotherapy with combination chemotherapy in gastric cancer, only very small portion of the patients underwent the treatment. PORT for high risk locally advanced gastric cancer resulted in excellent locoregional control with acceptable treatment-related morbidity, which was also comparable with other series. Postoperative CRT in addition to definitive resection and perioperative chemotherapy may provide improved treatment result in terms of locoregional control in these high risk patients. However, as these results are from small size retrospective series from single institution, suggestions from current studies should be validated with larger cohort from either large volume center or multiple small volume centers.
Notes
No potential conflict of interest relevant to this article was reported.