A patient who has survived for a long period with repeated radiotherapies for multifocal extrahepatic metastases from hepatocellular carcinoma
Article information
Abstract
Although significant advances in the treatment of intrahepatic lesions, it is reported that the prognosis for patients with hepatocellular carcinoma (HCC) who have extrahepatic metastasis remains poor. We report a patient with lung, liver, brain, bone and subcutaneous metastasis from HCC who has survived more than 7 years maintaining relatively good performance status as a result of repeated therapies. A 55-year-old male patient with HCC underwent right lobectomy of the liver and cholecystectomy in September 2006. He received wedge resection for lung metastasis twice (July 2009, January 2011) and Gamma Knife stereotactic radiosurgery for brain metastasis (April 2011). Over the last 3 years, he has developed metastasis in subcutaneous tissues, muscle, and bone with pain. He has undergone 7 courses of radiotherapies for subcutaneous tissues, muscle, and bone metastasis and been prescribed sorafenib and he is still capable of all self-care.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second most common cause of cancer deaths in Korea [1]. Recent advances in diagnostic modalities and therapeutic approaches have improved the prognosis for patients with HCC [2,3], and extrahepatic metastases are diagnosed more frequently due to prolonged survival. However, there is little information regarding the prognosis and effective treatment of patients with HCC and extrahepatic metastasis.
The aim of this report is to describe the case of a HCC patient with multiple extrahepatic metastases who has lived after seven courses of radiotherapy and review of the extrahepatic metastasis in literature.
Case Report
A 55-year-old man with hepatitis B and C was diagnosed with HCC in September 2006, and subsequently underwent a right lobectomy of the liver and cholecystectomy. HCC was multiple and the largest lesion was 7 cm. HCC was Edmondson-Steiner grade 3/3 and complicated by cirrhosis. There wasn't vascular invasion and resection margin was clear. During follow-up, lung metastasis was found and wedge resection was undergone twice (right lower lobe, July 2009; left lower lobe, January 2011). In March 2011, he felt pain on a palpable mass of his left shoulder. He visited local clinic and a biopsy of the mass revealed metastatic HCC. He was referred to our hospital in March 2011 for further evaluation and treatment. His Eastern Cooperative Oncology Group performance status was 1. Soft tissue metastases in the left shoulder area and abdominal wall were showed on computed tomography and positron emission tomography/computed tomography (PET/CT). There was no intrahepatic lesion and α-fetoprotein (AFP) was normal. And Child-Pugh score was 5 (Class A).
1. 1st radiotherapy (April 2011, 2 sites): left pectoralis major and deltoid muscle area/left teres minor muscles area
Huge hard fixed masses were detected on his left pectoralis major, deltoid, and left teres minor muscles in which metastatic HCC was confirmed (Fig. 1A, B). Radiotherapy was delivered 35 Gy in 14 fractions for each lesion. After radiotherapy, back mass was softened and its size decreased. The masses of pectoralis major and deltoid muscle were also decreased (Fig. 1C). The brain metastasis was detected during the radiotherapy. He underwent Gamma Knife (Elekta AB, Stockholm, Sweden) stereotactic radiosurgery and started to take sorafenib (200 mg/day) in other hospital.
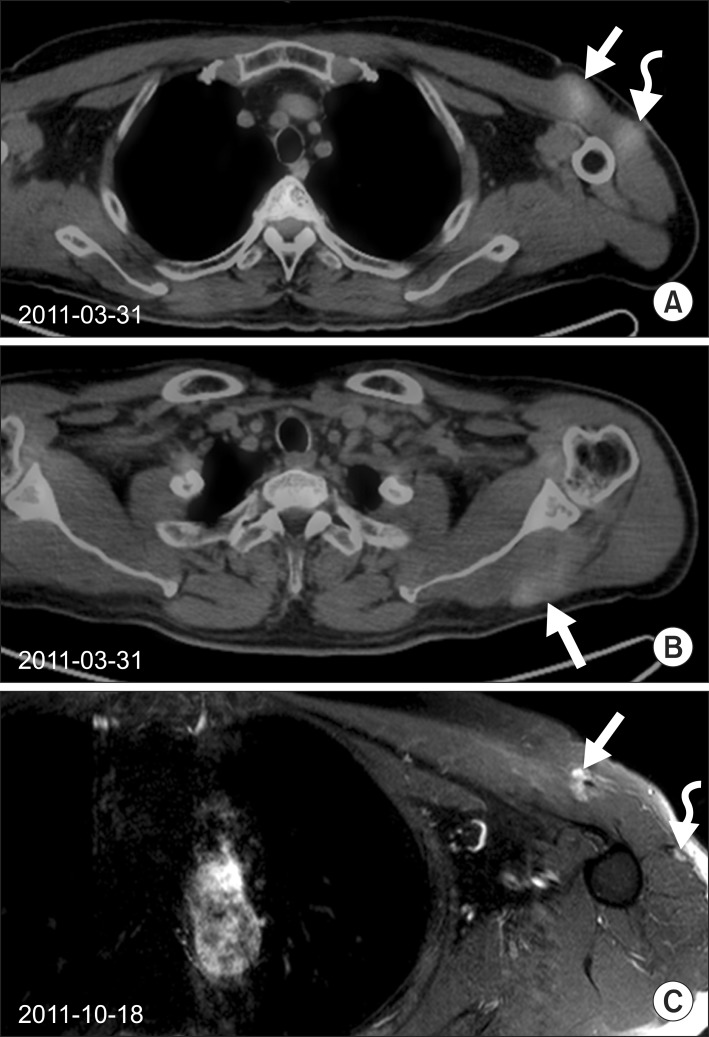
(A) Two well-enhanced intramuscular masses (3.5 cm × 1.8 cm; 2.2 cm × 1.9 cm) in the left pectoralis major (arrow) and left deltoid muscles (curved arrow) were visualized on PET-CT (maxSUV 2.57, 2.30) in March 2011. (B) An intramuscular mass along the left teres minor muscle was also visualized on PET/CT (maxSUV 2.00) in March 2011. Radiotherapy of 35 Gy in 14 fractions was delivered. (C) After radiotherapy of 35 Gy in 14 fractions, these masses were decreased in size (1.5 cm × 1.2 cm) markedly. PET/CT, positron emission tomography/computed tomography.
2. 2nd radiotherapy (July and August 2011, 1 site): abdominal wall
Due to about 6-cm sized painful abdominal mass, he underwent radiotherapy in another hospital. Radiotherapy was delivered 40 Gy in 16 fractions.
3. 3rd radiotherapy (November 2011, 1 site): left teres minor muscle area (Fig. 2A, B)
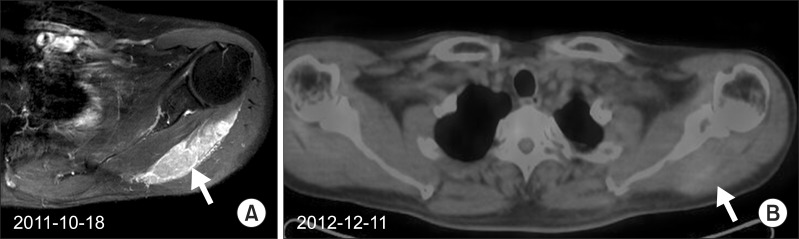
(A) A hard fixed mass in left teres minor muscles area was palpable again on T1 gadolinium-enhanced magnetic resonance in October 2011. Subsequent radiotherapy of 30 Gy in 12 fractions was delivered over 3-week period. (B) Grossly the metabolism of the mass was decreased on the outside positron emission tomography/computed tomography in December 2012.
Though left pectoralis major and deltoid lesions nearly disappeared and pain was improved after radiotherapy, pain of left teres minor muscles area was aggravated and hard fixed mass was palpable again. A total dose of 30 Gy in 12 fractions for the left teres minor muscle area was delivered over a 3-week period.
4. 4th radiotherapy (June 2012, 2 sites): abdominal wall
Two painful recurred hard fixed masses were detected on abdominal wall. A total dose of 30 Gy in 15 fractions (Fig. 3A, B) and 27 Gy in 9 fractions were delivered for each lesion. The masses showed decreased metabolism grossly on outside PET/CT in December 2012. In January 2013 he received excision of the abdominal wall mass due to aggravated pain. In February 2013, a recurred abdominal mass was detected again and another excision was undergone because of previous radiotherapy history.
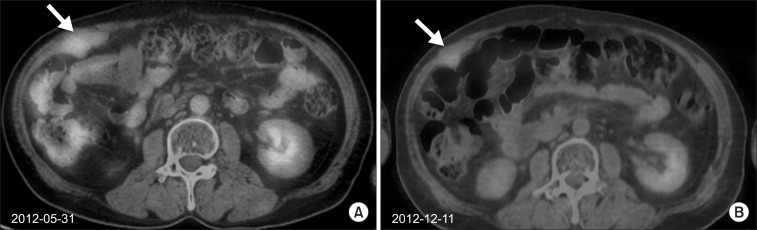
(A) A fluorodeoxyglucose (FDG)-avid well-enhanced conglomerated recurred muscular mass (5.0 cm × 2.7 cm) was detected in the right abdominal wall on PET/CT (maxSUV 2.84) in May 2012. (B) Since a total dose of 30 Gy in 15 fractions were delivered, the mass showed decreased metabolism grossly on outside PET/CT in December 2012. PET/CT, positron emission tomography/computed tomography.
5. 5th radiotherapy (July 2012, 1 sites): left acromial angle area (Fig. 4A, B)

(A, B) In July 2012, he felt pain on his left shoulder, there was newly developed well-enhanced lesion in his left acromion on T1 gadolinium-enhanced magnetic resonance (arrow). After receiving radiotherapy of 20 Gy in 5 fractions, his pain was relieved.
He felt pain on his left shoulder, new metastatic lesion in acromial angle was detected. He received radiotherapy of 20 Gy in 5 fractions. Pain was improved after radiotherapy.
6. 6th radiotherapy (October and November 2012, 2 sites): scalp, right deltoid muscle area (Fig. 5A, B)
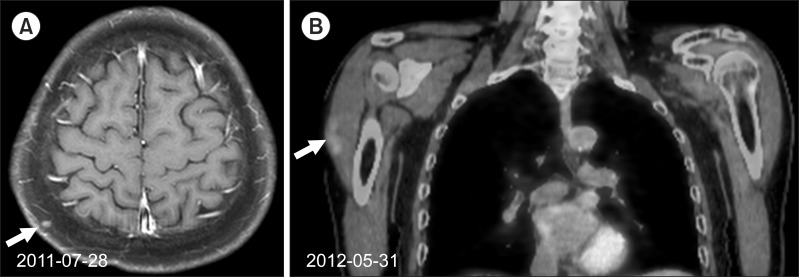
(A) In October 2012, about 1-cm sized painful scalp mass was detected. His previous T1 gadolinium-enhanced magnetic resonance showed about 0.3-cm sized well-enhanced nodule in the right posterior scalp area in July 2011. The excision was undergone. It was pathologically reported as metastatic hepatocellular carcinoma. (B) He also felt a newly growing mass in his right upper arm in May 2012. Positron emission tomography/computed tomography showed a hypermetabolic lesion in his right deltoid muscle (maxSUV 2.00). A total dose of 35 Gy in 14 fractions each was delivered for the tumor bed of his scalp and the right upper arm, and the pain was relieved.
A small scalp mass was detected, and excisional biopsy was performed on October 2012. The pathology of the lesion was metastatic HCC. He also felt a newly growing mass in his right arm. Radiotherapy was undergone for the tumor bed of his scalp and the right deltoid muscle area. A total dose of 35 Gy in 14 fractions was delivered for each lesion, and the pain was relieved.
7. 7th radiotherapy (July 2013, 1 site): left pectoralis major muscle area
A 5-cm sized rapid-growing hard fixed mass was detected with pain on his left anterior chest wall again. Radiotherapy of 35 Gy in 14 fractions was delivered, and pain was improved.
The patient underwent 7 courses of radiotherapy and took sorafenib orally (at starting dosage of 200 mg/day and a maintaining dosage of 600 mg/day) from April 2011 to July 2013. His hepatic function has been well preserved and the level of AFP has been normal. The brain metastasis had been also stable. Lung metastasis was suspicious on chest CT but that had not been progressed though intramuscular lesions were progressed after radiotherapy. He is still alive and is possible to carry on ordinary activities.
Discussion and Conclusion
Extrahepatic metastasis occurs in about 15%-37% of patients with HCC, and it depends on HCC stages [4-6]. The most frequent sites of metastases in HCC patients are the lungs, lymph nodes, bones, and adrenal glands [4-7]. Subcutaneous or muscular metastasis has been reported rarely. At the initial diagnosis of extrahepatic metastases, many HCC patients have been reported to have advanced intrahepatic stage [4,5,7]. Subcutaneous metastases of HCC are mostly due to iatrogenic causes, such as those associated with needle tract implantation. The reason why non-iatrogenic subcutaneous metastases of HCC are rare is not known. Cases with subcutaneous metastasis with normal AFP level in the serum are extremely rare [8]. According to study of Huang et al. [9], the average latency period for the occurrence of the subcutaneous metastases was 291 days and the scalp was the most frequent metastatic site.
Despite significant advances in the treatment of intrahepatic lesions, the prognosis for patients with HCC who have extrahepatic metastasis remains poor. Natsuizaka et al. [7] showed that the median survival time and 1-year survival rate were 7 months (range, 1 to 59 months) and 24.9%, respectively. In another Japanese study, the cumulative survival rates after the initial diagnosis of extrahepatic metastases at 6, 12, 24, and 36 months were 44.1%, 21.7%, 14.2%, and 7.1%, respectively, and the median survival time was 4.9 months (range, 0 to 37 months) [5]. The major cause of death in patients with HCC who have extrahepatic metastases is progression of the intrahepatic HCC lesion. Uka et al. [5] showed that 14 patients (11%) among 126 HCC patient with extrahepatic metastasis died of extrahepatic HCC, others died of primary HCC or liver failure. The control of intrahepatic lesions and performance status were identified as important prognostic factors in patients with advanced HCC who had extrahepatic metastasis [6,10]. Jung et al. [10] showed that Child-Pugh class A, smaller hepatic tumor size, absence of portal venous invasion, single metastatic organ involvement, and objective treatment response of the intrahepatic tumor were the favorable prognostic factors for survival. Similarly, Natsuizaka et al. [7] revealed that patients with Child-Pugh grade B and C (p = 0.0018) and patients with serum AFP >40 ng/mL (p = 0.011) had significantly poor prognosis.
There is no standard treatment for extrahepatic metastases of primary HCC. Huang et al. [9] reported 21 patients with a subcutaneous HCC metastasis and without seeding who were treated by radiotherapy. Radiotherapy was found to result in a satisfactory treatment response for HCC patients with subcutaneous metastasis. For bone metastasis from HCC, radiotherapy also showed good response (73%) in relief of pain [11]. Resection of metastatic lesions produced a satisfactory local response [6]. Kim et al. [12] demonstrated that sorafenib resulted in superior survival in patients with extrahepatic metastasis (hazard ratio = 0.539, p = 0.003).
Niibe et al. [13,14] proposed the new notion of oligo-recurrence, one to several distant metastasis/recurrences in one to several organs with primary site controlled, that is treatable with local therapy. According to the definition, the patient was in compatible with oligo-recurrence when he visited our hospital at first. Punglia et al. [15] reported that improvement of systemic chemotherapy including molecular-targeted therapy has allowed micrometastases to be almost curable, the role of local therapy for gross metastatic or recurrent sites grows bigger. Niibe and Hayakawa [16] suggested that all the gross tumors could be treated with local therapy in the state of oligo-recurrence, meaning curative treatment. Multidisciplinary approach to oligo-recurrence is important and more studies are needed.
There has been little study regarding HCC with oligo-recurrence and no evidence of proper regimen of radiotherapy. We delivered 35 Gy in 14 fractions to this patient's shoulder for palliation of pain because of his previous history of multiple metastases. However, he needed re-irradiation for the same lesion, the dose was resultingly suboptimal. Moreover, the patient received treatments in three different hospitals and more effective multidisciplinary approach was difficult to apply.
Similarly to this case, there was a case report of long-term survival of a patient with multiple abdominal metastases from endometrial carcinoma treated with multi-portal conformal re-irradiation and chemotherapy [17]. Yun [18] also reported a gastric cancer patient with multiple metastases survived for 4 years after radiotherapies to 17 metastases. However, cases of multiple metastatic HCC who has survived for over 7 years as our patient have been rarely reported. For 3 years, though his extrahepatic metastasis progressed, there was no intrahepatic metastasis. Liver function was good and the level of AFP was normal. His performance status was relatively good though he needed supportive care for pain. Conclusively, more aggressive local treatment for extrahepatic metastasis should be considered for HCC patients with extrahepatic metastasis who had controlled intrahepatic disease and good performance status.
Notes
No potential conflict of interest relevant to this article was reported.